May 14 2019
Researchers from the Eindhoven University of Technology (TU/e) have found that the stability of perovskite solar cells increases considerably when a small amount of fluoride is added to them.
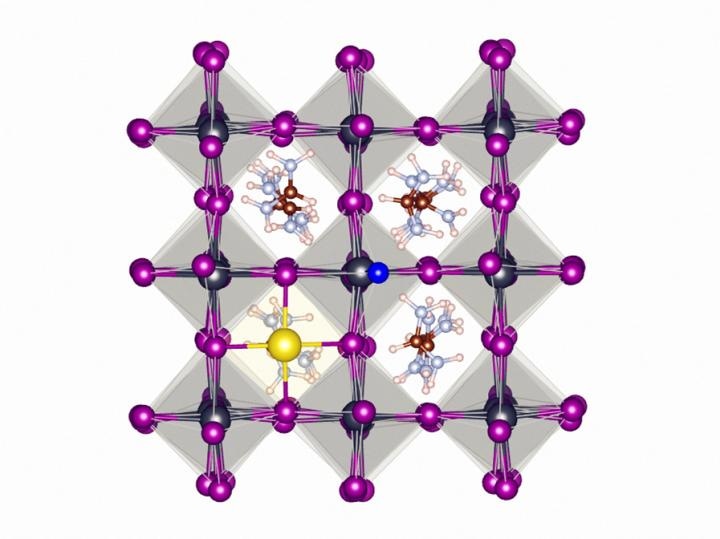 The atomic structure of fluoride (NaF) containing metal halide perovskite (FAPbI3). Due to its high eletronegativity, fluoride stabilizes the perovskite lattice by forming strong hydrogen bonds and ionic bonds on the surface of the material. (Image credit: Eindhoven University of Technology)
The atomic structure of fluoride (NaF) containing metal halide perovskite (FAPbI3). Due to its high eletronegativity, fluoride stabilizes the perovskite lattice by forming strong hydrogen bonds and ionic bonds on the surface of the material. (Image credit: Eindhoven University of Technology)
Perovskite-based solar cells hold immense potential for the future of solar energy. This is because perovskite materials are low-cost, easy to create, and nearly as efficient as silicon—the material tipsily utilized in solar cells. Conversely, perovskite has a tendency to degrade rapidly, extremely restricting its stability and efficiency over time.
Now, scientists from the Eindhoven University of Technology, University of Twente, and Peking University have discovered that when a small quantity of fluoride is added to the perovskite, a protective layer is produced that considerably increases the stability of the solar cells and the materials. It was observed that the solar cells retained 90% of their efficiency following 1000 hours operation at numerous rigorous testing conditions. The results of the study have been recently reported in the leading scientific journal, Nature Energy.
Since perovskite solar cells can be produced cost-effectively, they have been at the center of intense solar research in the recent past. As a result, the efficiency of these solar cells has increased from less than 4% in 2009 to more than 24% at present, which is near to conventional silicon cells. In fact, an efficiency of over 28% is achieved by the so-called tandem cells, which integrate perovskite and silicon cells.
But in spite of this success, perovskite has several drawbacks owing to the way it is produced and the nature of the material. In due course, vacancies in the metal halide atomic structure prompt the degradation of the perovskite material under the effect of heat, light, and moisture.
Protective Layer
The research team from Twente, TU/e, and Beijing has experimented with a unique kind of perovskite, by introducing a small quantity of fluoride in the production process. Similar to the fluoride used in toothpastes, the fluoride ions create a protective layer over the crystal and thus prevent the diffusion of the destructive defects.
Our work has improved the stability of perovskite solar cells considerably. Our cells maintain 90 percent of their efficiency after 1000 hours under extreme light and heat conditions. This is many times as long as traditional perovskite compounds. We achieve an efficiency of 21.3 percent, which is a very good starting point for further efficiency gains.
Shuxia Tao, Study Co-Author and Assistant Professor, Center for Computational Energy Research, Department of Applied Physics, Eindhoven University of Technology
Most of the work carried out by the group from TU/e has explained why fluoride is a highly effective ingredient as opposed to other halogens. With the help of computer simulations, the researchers reached a conclusion that part of the fluoride’s success is due to the high electronegativity and tiny size of its ions.
When an element‘s electronegativity is higher, it attracts electrons of neighboring elements more easily. As a result, the fluoride ions are able to create strong bonds with the other elements present in the perovskite compound, thus producing a stable protective layer.
Future Research
This latest research is viewed as a major step towards the effective implementation of perovskite solar cells in the coming days. However, more research needs to be done. In the solar sector, the gold standard is a retention rate of a minimum of 85% of original efficiency after 10 to 15 years—a standard which is still a bit way off for perovskite solar cells.
We expect it will take another five to ten years for these cells to become a commercially viable product. Not only do we need to further improve their efficiency and stability, we also need to gain a better theoretical understanding of the relevant mechanisms at the atomic scale. We still don’t have all the answers to why some materials are more effective than others in increasing the long-term stability of these cells.
Shuxia Tao, Study Co-Author and Assistant Professor, Center for Computational Energy Research, Department of Applied Physics, Eindhoven University of Technology