Jun 26 2019
One mode through which heat ruins electronic equipment is by making components to expand at different rates, leading to forces that cause distortion and micro-cracking.
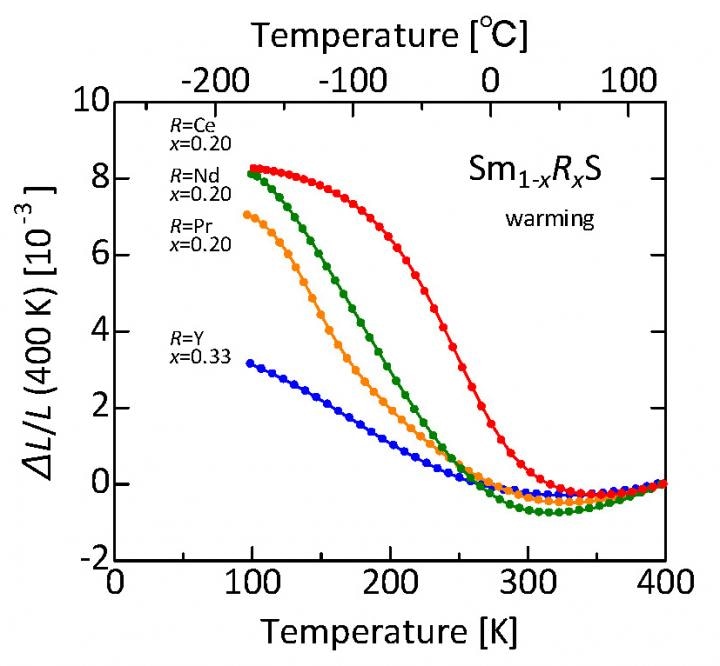 Samarium sulfide doped with various rare earth elements shrinks as the temperature increases from about minus 175 °C to about 40-60 °C. Shown here is the relative linear shrinkage compared to the length at about 120°C. For the Cerium (Ce) dopant, the percentage volume decrease is about 2.6%. These samples were produced by an industrially scalable process, paving the way for practical applications of this class of sulfides as thermal-expansion compensators. (Image credit: K. Takenaka/John Wojdylo)
Samarium sulfide doped with various rare earth elements shrinks as the temperature increases from about minus 175 °C to about 40-60 °C. Shown here is the relative linear shrinkage compared to the length at about 120°C. For the Cerium (Ce) dopant, the percentage volume decrease is about 2.6%. These samples were produced by an industrially scalable process, paving the way for practical applications of this class of sulfides as thermal-expansion compensators. (Image credit: K. Takenaka/John Wojdylo)
Plastic components and circuit boards are specifically susceptible to damage because of variations in volume during cooling and heating cycles. However, if a material could be integrated into the components that could counterbalance the expansion, the stresses would be decreased and their lifetime would be prolonged.
All are aware of one material that acts like this: liquid water expands on freezing and ice contracts on melting. However, electronics and liquid water are not a good combination, and instead a solid with “negative thermal expansion” (NTE) is what actually required.
While such materials have been identified since the 1960s, there are several obstacles that must be overcome before the concept would be widely useful and commercially feasible. With regard to materials as well as function, these attempts had only restricted success.
The experimental materials had been made under particular laboratory conditions using costly equipment; but still, the pressure and temperature ranges in which they would show NTE were well beyond typical everyday conditions. Furthermore, the amount they expanded and contracted was based on the direction, which caused internal stresses that modified their structure, implying that the NTE property would not persist longer than a few heating and cooling cycles.
A group of scientists headed by Koshi Takenaka of Nagoya University was successful in overcoming these materials-engineering issues. Drawing inspiration from the series of work by Noriaki Sato, also from Nagoya University—whose discovery of superconductivity in quasicrystals in 2018 was regarded as one of the top 10 physics discoveries of the year by Physics World magazine—Professor Takenaka employed the rare earth element samarium and its sulfide, samarium monosulfide (SmS), which transforms phase from the “black phase” to the smaller-volume “golden phase”.
The issue was to adjust the temperature range at which the phase transition takes place. The researchers’ solution was to substitute a small fraction of samarium atoms with another rare earth element, offering Sm1-xRxS, where “R” is any one of the rare earth elements neodymium (Nd), cerium (Ce), yttrium (Y), or praseodymium (Pr). The fraction “x” used by the research group was normally 0.2, excluding yttrium. These materials exhibited “giant negative thermal expansion” of up to 8% at normal room pressure and a practical range of temperatures (about 150 °C) including at room temperature and beyond. In this scenario, cerium is the principal candidate as it is comparatively low priced.
The characteristic of the phase transition is such that the materials can be broken up into extremely small crystal sizes about a micron on a side without losing their negative expansion property. This widens the industrial applications, specifically within electronics.
Although the engineering accomplishment by Nagoya University team is remarkable, how the negative expansion functions is interesting from a basic physics perspective. During the transition of the black-golden phase, the crystal structure remains the same but the atoms get closer together—that is, the unit cell size becomes smaller since (as is very likely but possibly not yet 100% certain) the electron structure of the samarium atoms modifies and renders them smaller—a process of intra-atomic charge transfer known as a “valence fluctuation” or “valence transition” within the samarium atoms.
My impression is that the correlation between the lattice volume and the electron structure of samarium is experimentally verified for this class of sulfides.
Koshi Takenaka, Professor, Nagoya University
More particularly, in the black (lower temperature) phase, the electron arrangement of the samarium atoms is (4f)6, which implies that their outermost shell has 6 electrons in the f orbitals (with s, p, and d orbitals filled); whereas the electronic configuration in the golden phase is (4f)5(5d)1, implying that an electron has moved from a 4f orbital into a 5d orbital. While a “higher” shell is beginning to be filled, it turns out—through a quirk of the Pauli Exclusion Principle—that the second case provides a smaller atom size, resulting in a smaller crystal size and negative expansion.
However, this is only a portion of the basic picture. Samarium sulfide and its doped offshoots—in the black phase—are insulators (they do not conduct electricity); whereas in the golden phase, they become conductors (that is, metals). This implies that at the time of the black-golden phase transition, the band structure of the entire crystal is having an effect on the valance transition within the samarium atoms.
The theoretical calculations for the doped samarium sulfides made by Professor Takenaka’s group had not been done by anybody; however, a previous theoretical study has denoted that when electrons move out of the f orbital of samarium atoms, they leave behind a “hole” that is positively charged and which itself interacts repulsively with holes in the conduction band of the crystal, influencing their exchange interaction. This turns out to be a cooperative effect that subsequently propels the valence transition in the samarium atoms. However, the actual mechanism is not well understood.
Still, the Nagoya University-led team’s success is one of engineering, not pure physics.
What is important for many engineers is the ability to use the material to reduce device failure due to thermal expansion. In short, in a certain temperature range - the temperature range in which the intended device operates, typically an interval of dozens of degrees or more—the volume needs to gradually decrease with a rise in temperature and increase as the temperature falls.
Koshi Takenaka, Professor, Nagoya University
Takenaka continued, "Of course, I also know that volume expansion on cooling during a phase transition [like water freezing] is a common case for many materials. However, if the volume changes in a very narrow temperature range, there is no engineering value. The present achievement is the result of material engineering, not pure physics."
Perchance, it can even be a sign of a new “golden” era for electronics.