Image Credit: The University of Texas at Austin.
A majority of the batteries are made up of either solid-state electrodes such as lithium-ion batteries for portable electronics, or composed of liquid-state electrodes, such as flow batteries for smart grids.
The UT scientists have designed a so-called “room-temperature all-liquid-metal battery” that offers the best of both worlds—solid-state and liquid-state batteries.
While solid-state batteries have a considerable capacity for energy storage, they normally experience a number of issues that eventually cause them to deteriorate over time and make them less efficient.
By contrast, liquid-state batteries can provide energy in a more efficient way, without the long-term degradation of solid-state devices; however, these batteries cannot meet the high energy requirements, or they need considerable resources to continuously heat the electrodes and maintain them in a molten state.
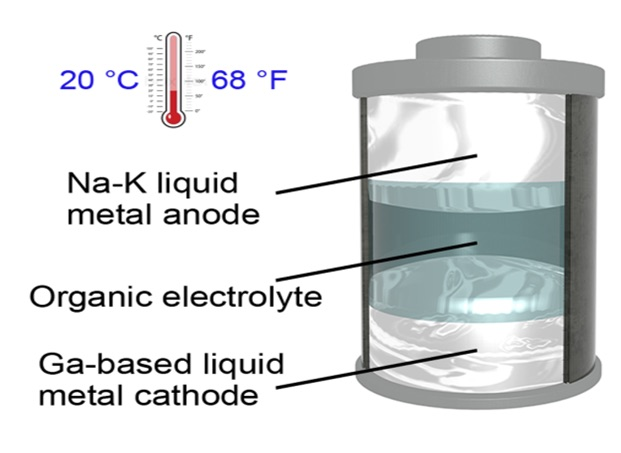
The metallic electrodes in the newly developed battery can remain in a liquid state at a temperature of 20 °C (68 °F)—the lowest operating temperature to be ever documented for a liquid-metal battery, stated the scientists. This denotes a significant change because present-day liquid-metal batteries should be maintained at temperatures over 240 °C.
This battery can provide all the benefits of both solid- and liquid-state—including more energy, increased stability and flexibility—without the respective drawbacks, while also saving energy.
Yu Ding, Study Lead Author and Postdoctoral Researcher, The University of Texas at Austin
Ding is also a part of associate professor Guihua Yu’s research team in the Walker Department of Mechanical Engineering. The article on the room-temperature battery was recently published in the Advanced Materials journal.
The battery contains a gallium-based alloy as the cathode and a sodium-potassium alloy as the anode. In the article, the team describes that with the help of different materials, a battery with even lower melting points could be developed.
The room-temperature battery could deliver more power when compared to the current generation of lithium-ion batteries, which are considered the backbone of many personal electronics. According to the team, the battery can charge and provide energy many times faster.
Due to the liquid components, the battery can be easily scaled up or down, based on the power required. If the battery is bigger, it can deliver more power. That kind of flexibility enables such batteries to possibly power everything, right from watches and smartphones to the infrastructure underpinning the transition toward renewable energy.
We are excited to see that liquid metal could provide a promising alternative to replace conventional electrodes. Given the high energy and power density demonstrated, this innovative cell could be potentially implemented for both smart grid and wearable electronics.
Guihua Yu, Associate Professor, Walker Department of Mechanical Engineering, The University of Texas at Austin
Despite spending over three years in this study, the team is yet to complete the job. Several elements that make up the backbone of this novel battery are more abundant when compared to some of the major materials used in conventional batteries, rendering them possibly easier and less costly to produce on a commercial level. But gallium continues to remain a costly material.
Discovering other materials that can provide the same level of performance while decreasing the production cost remains a major challenge.
The subsequent step to boost the power of the room-temperature battery involves enhancing the electrolytes—the parts that enable the electrical charge to pass via the battery.
Although our battery cannot compete with high-temperature, liquid-metal batteries at the current stage, better power capability is expected if advanced electrolytes are designed with high conductivity.
Yu Ding, Study Lead Author and Postdoctoral Researcher, The University of Texas at Austin
Journal Reference:
Ding, Y., et al. (2020) Room-Temperature All-Liquid-Metal Batteries Based on Fusible Alloys with Regulated Interfacial Chemistry and Wetting. Advanced Materials. doi.org/10.1002/adma.202002577.