Jan 29 2021
Scientists have used X-ray tomography to observe how the materials within solid-state lithium batteries evolve internally, as these batteries are charged and discharged simultaneously.
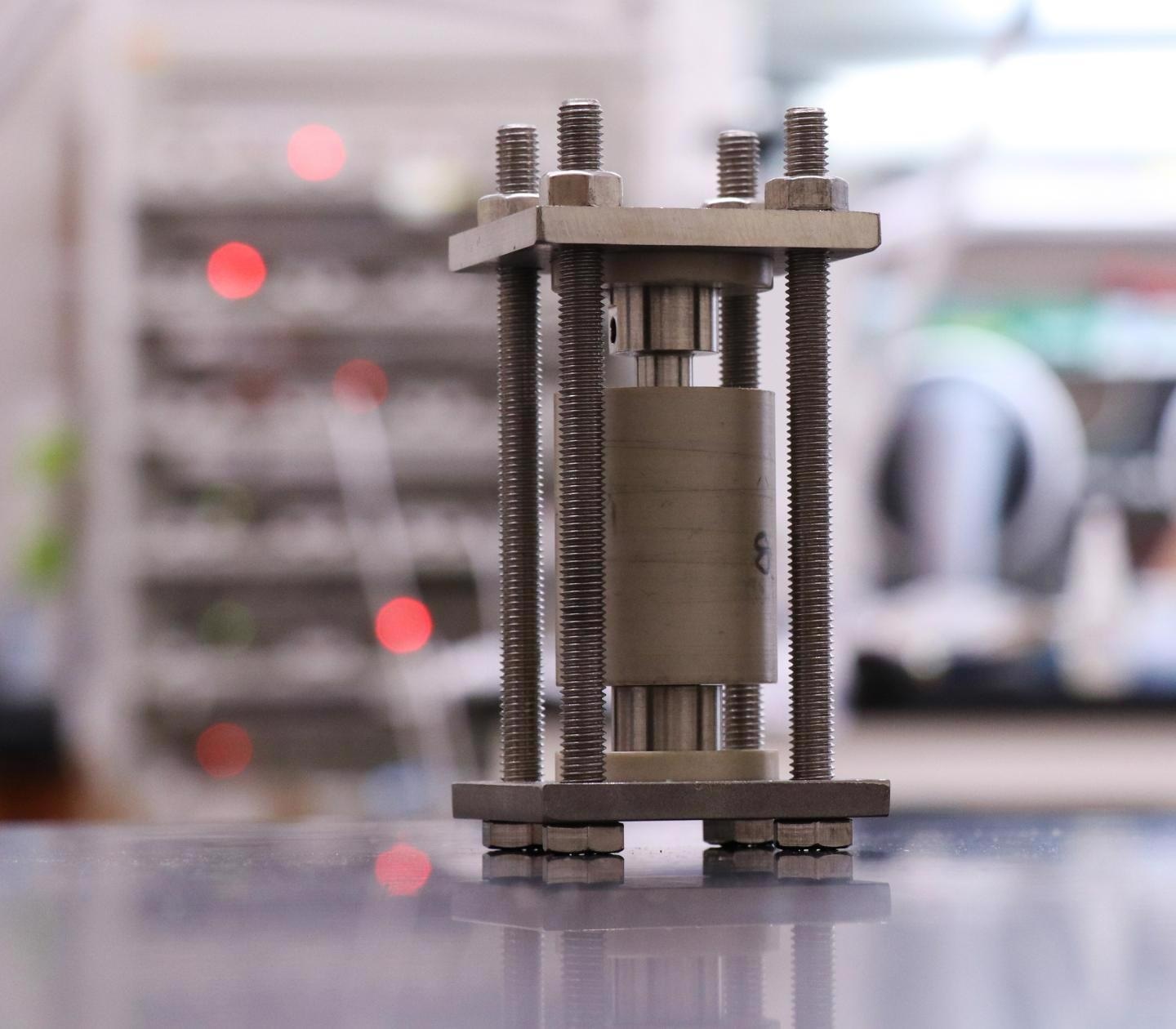 Solid-state batteries are charged and discharged in custom-made hardware designed at Georgia Tech. A smaller, modified version of the cell shown here was used to image these materials during cycling. Image Credit: Matthew Mcdowell, Georgia Institute of Technology.
Solid-state batteries are charged and discharged in custom-made hardware designed at Georgia Tech. A smaller, modified version of the cell shown here was used to image these materials during cycling. Image Credit: Matthew Mcdowell, Georgia Institute of Technology.
In-depth 3D data from the study could help enhance the performance and reliability of the batteries, which utilize solid materials to substitute the flammable liquid electrolytes in currently available lithium-ion batteries.
The novel operando synchrotron X-ray computed microtomography imaging showed how the behavior of solid-state batteries is determined by the dynamic variations of electrode materials at the interfaces of lithium and solid electrolytes.
The investigators observed that the operation of the batteries resulted in the formation of voids at the interface, which led to a loss of contact that mainly caused the failure in the cells.
This work provides fundamental understanding of what is happening inside the battery, and that information should be important for guiding engineering efforts that will push these batteries closer to commercial reality in the next several years.
Matthew McDowell, Assistant Professor, George W. Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, Georgia Institute of Technology
McDowell added, “We were able to understand exactly how and where voids form at the interface, and then relate that to battery performance.”
Funded by the National Science Foundation, a Sloan Research Fellowship, and the Air Force Office of Scientific Research, the study will be reported in the Nature Materials journal on January 28th, 2021.
The lithium-ion batteries, which are now extensively used for everything from electric vehicles to mobile electronics, depend on a liquid electrolyte to transport ions to and fro between electrodes inside the battery during the charge-discharge cycles. The liquid electrolyte evenly coats the electrodes, enabling the ions to move freely.
Instead, a solid electrolyte is used by rapidly-evolving solid-state battery technology, and it is believed that this electrolyte would help increase energy density and enhance the safety aspects of upcoming batteries.
However, when lithium is removed from electrodes, voids can form at the interface, causing reliability problems that tend to reduce the operation time of the batteries.
To counter this, you could imagine creating structured interfaces through different deposition processes to try to maintain contact through the cycling process. Careful control and engineering of these interface structures will be very important for future solid-state battery development, and what we learned here could help us design interfaces.
Matthew McDowell, Assistant Professor, George W. Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, Georgia Institute of Technology
The researchers from Georgia Institute of Technology (Georgia Tech), headed by a graduate student and the first author of the study Jack Lewis, constructed unique test cells that measured around 2 mm wide and were developed to be examined at the Advanced Photon Source—a synchrotron facility at the Argonne National Laboratory, a U.S. Department of Energy Office of Science facility situated close to Chicago.
Four team members examined the differences in the structure of the battery during five days of rigorous experiments.
The instrument takes images from different directions, and you reconstruct them using computer algorithms to provide 3-D images of the batteries over time. We did this imaging while we were charging and discharging the batteries to visualize how things were changing inside the batteries as they operated.
Matthew McDowell, Assistant Professor, George W. Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, Georgia Institute of Technology
Since lithium is extremely light, it can be difficult to image this element with X-rays and needed a unique design of the test battery cells. The technology utilized at the Argonne National Laboratory is almost the same as that utilized for medical computed tomography (CT) scans.
“Instead of imaging people, we were imaging batteries,” McDowell added.
Due to limitations in the testing, the team was only able to visualize the battery structure via one cycle. In upcoming studies, McDowell would like to find out what exactly takes place across extra cycles, and whether the structure in some way acclimatizes to the formation and filling of voids.
According to the team, the results would probably apply to other formulations of electrolytes, and that the characterization method could be applied to achieve data relating to other battery processes. In the case of electric vehicles, battery packs should be able to tolerate a minimum of 1000 cycles during an estimated lifetime of 150,000 miles.
Although solid-state batteries integrated with lithium metal electrodes can provide additional energy for specified battery size, that benefit will not resolve the prevailing technology, unless they can offer similar lifetimes.
“We are very excited about the technological prospects for solid-state batteries. There is substantial commercial and scientific interest in this area, and information from this study should help advance this technology toward broad commercial applications,” concluded McDowell.
Journal Reference:
Lewis, J. A., et al. (2021) Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nature Materials. doi.org/10.1038/s41563-020-00903-2.