Feb 10 2021
Scientists at Rice University have developed a “defective” catalyst that makes the synthesis of hydrogen peroxide from oxygen simple.
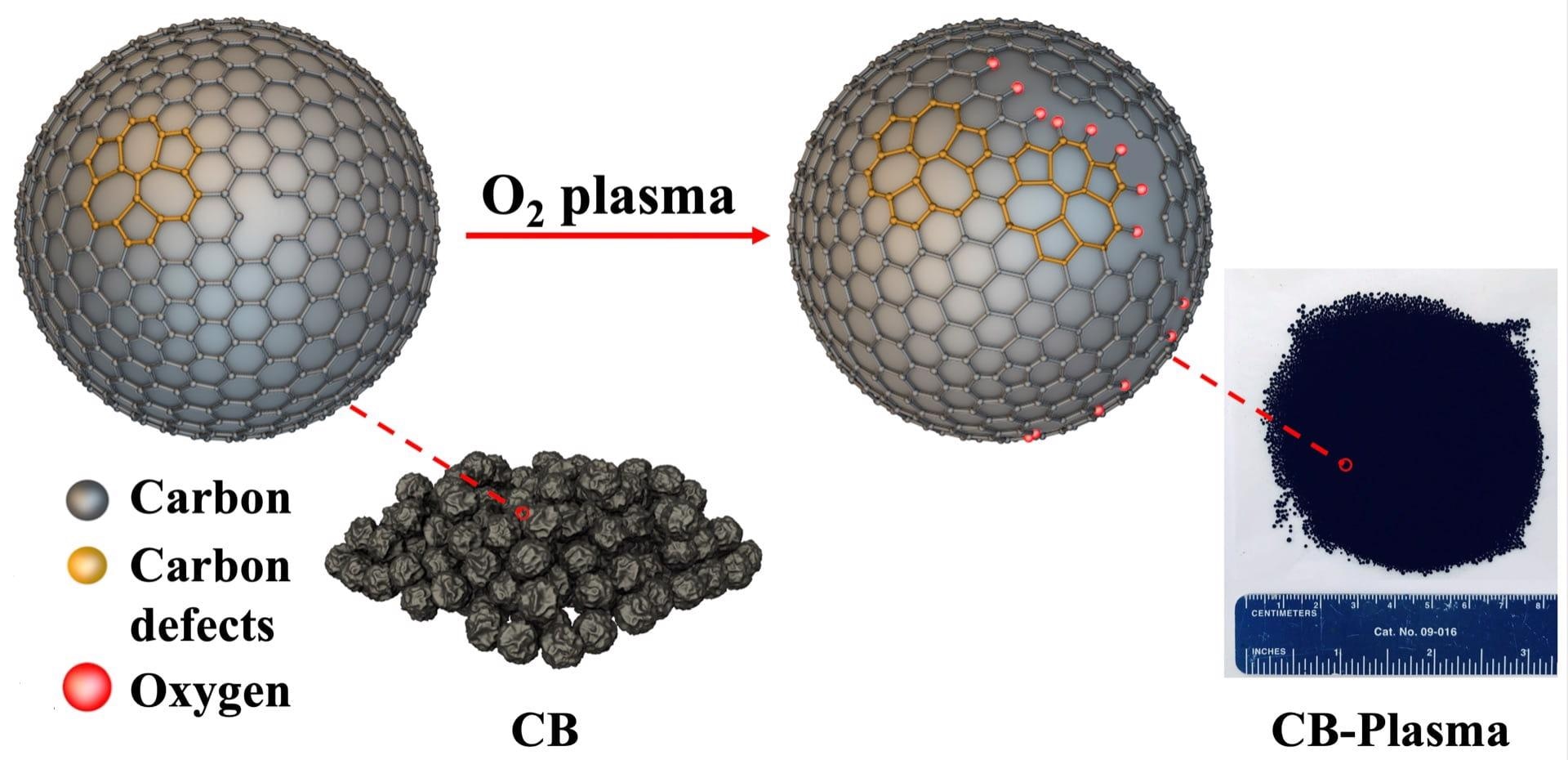 Scientists at Rice University have introduced plasma-treated carbon black as a simple and highly efficient catalyst for the production of hydrogen peroxide. Defects created in the carbon provide more catalytic sites to reduce oxygen to hydrogen peroxide. Image Credit: The Tour Group/Yakobson Research Group.
Scientists at Rice University have introduced plasma-treated carbon black as a simple and highly efficient catalyst for the production of hydrogen peroxide. Defects created in the carbon provide more catalytic sites to reduce oxygen to hydrogen peroxide. Image Credit: The Tour Group/Yakobson Research Group.
The researchers used oxygen plasma to treat metal-free carbon black—the cost-effective, powdered product of petroleum production. Through this process, defects and oxygen-containing groups are introduced into the structure of the carbon particles, thereby opening up more surface area for interactions.
When the defective particles called CB-Plasma are used as a catalyst, they reduce oxygen to hydrogen peroxide with a Faradaic efficiency of 100%. The Faradaic efficiency is a measure of the transfer of charges in electrochemical reactions.
The new process exhibits the potential to substitute the complex anthraquinone-based production technique that necessitates high-cost catalysts and produces toxic organic byproducts and huge amounts of wastewater, the researchers say.
The study by Rice chemist James Tour and materials theorist Boris Yakobson has been published in ACS Catalysis, an American Chemical Society journal.
Hydrogen peroxide is not only used extensively as a disinfectant but also for wastewater treatment, in the paper and pulp industries, as well as for chemical oxidation. Tour hopes that the new process will have an impact on the design of hydrogen peroxide catalysts in the future.
The electrochemical process outlined here needs no metal catalysts, and this will lower the cost and make the entire process far simpler. Proper engineering of carbon structure could provide suitable active sites that reduce oxygen molecules while maintaining the O-O bond, so that hydrogen peroxide is the only product. Besides that, the metal-free design helps prevent the decomposition of hydrogen peroxide.
James Tour, Chemist, Rice University
Plasma processing helps form defects in carbon black particles that look like five- or seven-member rings in the atomic lattice of the material. At times, the process eliminates sufficient atoms to form vacancies in the lattice.
The catalyst functions by removing two electrons from oxygen, thus enabling it to combine with two hydrogen electrons to produce hydrogen peroxide. (When oxygen is reduced by four electrons, a process employed in fuel cells, water is produced as a byproduct.)
The selectivity towards peroxide rather than water originates not from carbon black per se but, as (co-lead author and Rice graduate student) Qin-Kun Li’s calculations show, from the specific defects created by plasma processing. These catalytic defect sites favor the bonding of key intermediates for peroxide formation, lowering the reaction barrier and accelerating the desirable outcome.
Boris Yakobson, Materials Theorist, Rice University
At Tour’s lab, carbon black was also treated with ultraviolet-ozone and CB-Plasma was treated with argon after oxygen reduction to eliminate most of the oxygen-containing groups.
Although CB-UV was not better than plain carbon black in performing catalysis, CB-Argon performed as well as CB-Plasma with a more extensive range of electrochemical potential, according to the lab.
Since most of the oxygen groups were eliminated upon exposure of the CB-Plasma to argon under high temperature, the lab concluded that the carbon defects were themselves responsible for the catalytic reduction to hydrogen peroxide.
The simple nature of the process could enable more local generation of the useful chemical, thus decreasing the need to ship it from centralized plants. According to Tour, the efficiency of CB-Plasma matches that of most advanced materials that are currently used to produce hydrogen peroxide.
Scaling this process is much easier than present methods, and it is so simple that even small units could be used to generate hydrogen peroxide at the sites of need.
James Tour, Chemist, Rice University
The process is the second one to be launched by Rice in recent months to make hydrogen peroxide production more efficient. Haotian Wang, a Rice chemical and biomolecular engineer, and his lab created an oxidized carbon nanoparticle-based catalyst that generates the chemical from air, water and sunlight.
Zhe Wang, a Rice graduate student, is the co-lead author of the study. Other co-authors of the study are Rice alumnus Chenhao Zhang; graduate students Zhihua Cheng, Weiyin Chen, and Emily McHugh; and undergraduate Robert Carter.
Tour is the T.T. and W.F. Chao Chair in Chemistry and a professor of computer science and of materials science and nanoengineering. Yakobson is the Karl F. Hasselmann Professor of Materials Science and NanoEngineering and a professor of chemistry.
This study was supported by the Air Force Office of Scientific Research and the Office of Naval Research.
Journal Reference:
Whang, Z., et al. (2021) Hydrogen Peroxide Generation with 100% Faradaic Efficiency on Metal-Free Carbon Black. ACS Catalysis. doi.org/10.1021/acscatal.0c04735.