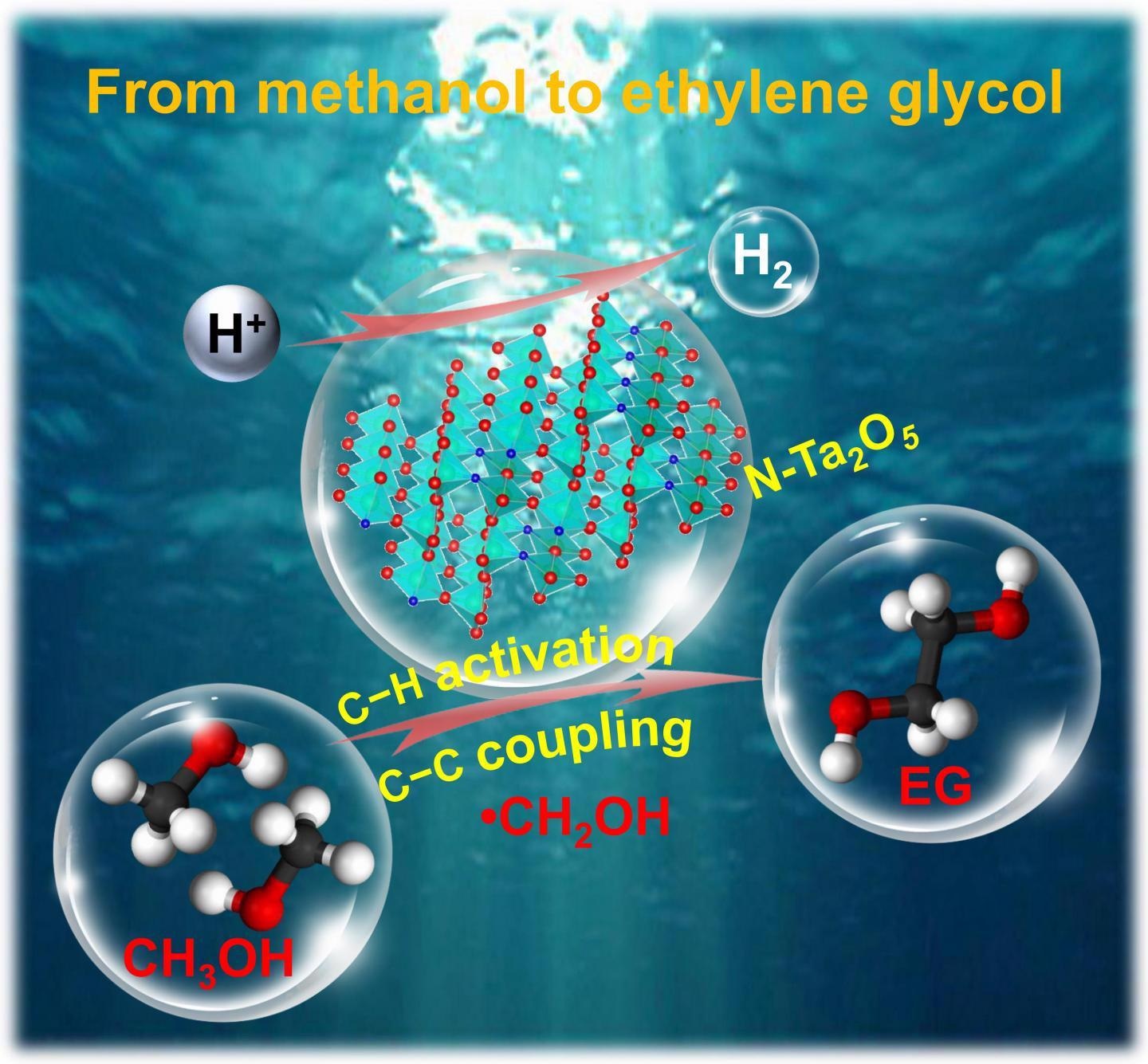
Direct photocatalytic coupling of methanol to ethylene glycol (EG) is highly attractive. The first metal oxide photocatalyst, tantalum-based semiconductor, is reported for preferential activation of C-H bond within methanol to form hydroxymethyl radical (CH2OH) and subsequent C-C coupling to EG. The nitrogen-doped tantalum oxide (N-Ta2O5) photocatalyst is an environmentally friendly and highly stable candidate for photocatalytic coupling of methanol to EG. Image Credit: Chinese Journal of Catalysis.
Photochemistry has been useful in regulating several reaction processes, particularly for the complicated reactions that include selective C-C coupling and C-H activation in chemical synthesis.
It is of great importance that a “dream catalytic reaction” of direct coupling of methanol to ethylene glycol (2CH3OH + HOCH2CH2OH + H2, denoted as MTEG) can be realized by the solar energy-driven C-C coupling and C-H activation processes, and still, this MTEG reaction has not been obtained through thermocatalysis.
Ethylene glycol (EG) is considered an essential monomer for manufacturing polymers (for example, poly (ethylene terephthalate), PET) and can also be utilized as a fuel additive and antifreeze. The EG’s yearly production is over 25 million tons, which is mainly generated from petroleum-derived ethylene industrially.
Methanol is one of the clear platform chemicals, which can not only be generated conventionally from coal and natural gas but also has been synthesized instantly from CO2 and biomass. Therefore, an alternative process for sustainable synthesis of H2 and EG from methanol instantly with huge attractions has been provided by the solar energy-driven MTEG route.
Despite the fact that direct photocatalytic coupling of methanol to EG is attractive enough, the stated photocatalysts for this reaction are all metal sulfide semiconductors, which might undergo photo corrosion and possess low stability. Hence, the growth of non-sulfide photocatalysts for effective photocatalytic coupling of methanol to EG and H2 with high stability tends to be crucial but also extremely difficult.
A research group headed by Professor Ye Wang from Xiamen University and Yanshan University, China, has proposed the first metal oxide photocatalyst, tantalum-based semiconductor, for selective activation of C-H bond inside methanol to develop hydroxymethyl radical (CH2OH) and following C-C coupling to EG.
In contrast to other metal oxide photocatalysts, like Nb2O5, WO3, ZnO, and TiO2, tantalum oxide (Ta2O5) is considered to be special because it can achieve the selective photocatalytic coupling of methanol to EG.
The co-catalyst-free nitrogen-doped tantalum oxide (2%N-Ta2O5) exhibits an EG formation rate as high as 4.0 mmol/g/h, approximately nine times higher compared to Ta2O5, with a selectivity greater than 70%.
The high charge separation potential of nitrogen-doped tantalum oxide plays a major role in its high activity for the synthesis of EG. Moreover, this catalyst exhibits outstanding stability for more than 160 hours, which has not been realized over the reported metal sulfide photocatalysts.
Tantalum-based photocatalyst is considered to be a highly stable and eco-friendly candidate for photocatalytic coupling of methanol to EG.
Journal Reference:
Wang, L., et al. (2021) Solar energy-driven C−H activation of methanol for direct C−C coupling to ethylene glycol with high stability by nitrogen doped tantalum oxide. Chinese Journal of Catalysis. doi.org/10.1016/S1872-2067(21)63797-X.