Polaritons are hybrid particles comprised of a photon and an electric dipole that are highly linked. Polaritons are quasiparticles that form when electromagnetic waves are strongly coupled to a magnetic or electric dipole-carrying stimulation.
They are an example of level attraction, a typical quantum phenomenon known as the avoidance crossing principle. An electron-hole pair in semiconductors, which generates an exciton-polariton, and vibrating electrons at the metal's surface, which generates a surface plasmon polariton, are examples of such dipoles.
Light-Matter Coupling
Over the last ten years, significant theoretical and experimental work has been made to show that strong light-matter coupling may be used to affect molecular energy environments and enhance energy transfers in donor-acceptor molecular structures, among other things. Strong coupling occurs when a group of quantum transmitters is put inside an optical cavity because the consistent transfer of energy between both the emitters' excitons and photons within the cavity is quicker than the combined system's loss mechanisms.
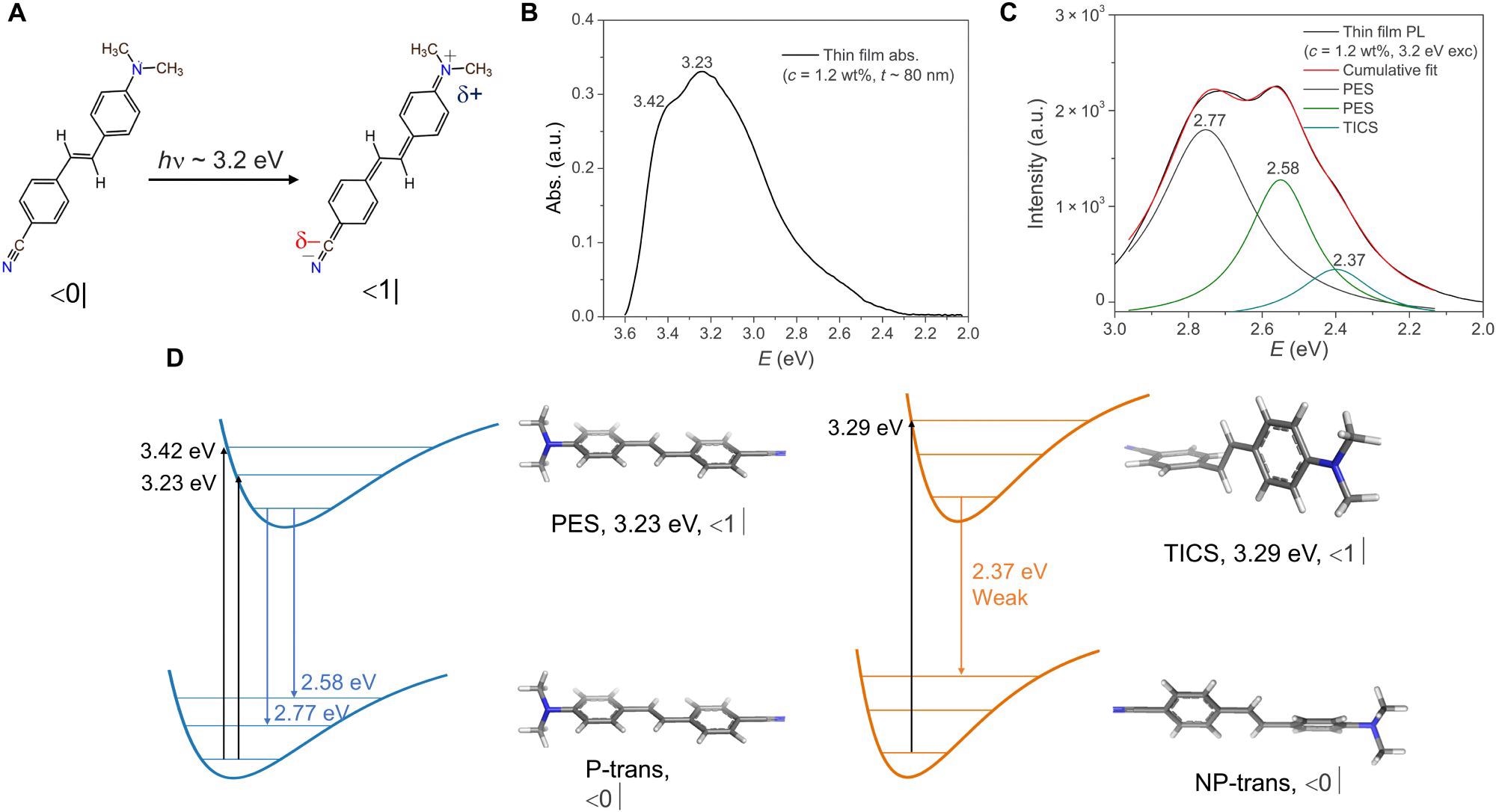
Optical characterization of the thin film sample. (A) The ground (|0>) and the excited ICT state (|1>) chemical structures of trans-DCS, (B) absorption and (C) PL plots (the deconvoluted peaks assign the spectral weights for the respective PES and TICS exciton emission), and (D) schematic comparing the respective absorption and emission energies of the ground (P and NP) and excited state (PES and TICS) configurations of DCS (c = 1.2 wt%) thin film (~80 nm) over Ag. a.u., arbitrary units. Image Credit: Sataphaty, S., et al., Science Advances
The creation of novel hybrid light-matter states commonly referred to as polaritonic states, which inherit fascinating features from their two main components, matter and light, and have a delocalized nature, is the most notable fingerprint of strong coupling.
That is because organic materials have promising importance in the area of strong coupling because they produce exceptionally robust polaritonic states under ambient settings with massive Rabi splitting up to 1 eV due to their high densities, huge dipole moments, and firmly bound Frenkel excitons.
A method for selecting isomer luminescence that also uses the collective strong light-matter coupling phenomena is exciton-polariton funneling. The process is based on the excitation being funneled through the collective polaritonic mode, which takes advantage of its delocalized nature.
The polaritonic mode that results from picking an organic molecule with two isomers with a highly similar absorption spectrum contains contributions from two excited states of the isomers present in the solution. This property correlates to photon emissions from a molecule's isoenergetic Twisted ICT Excited State (TICS), meaning that the proportion of NP-trans isomers in our DCS films grows as concentration increases. In contrast to the PL spectrum, absorption does not vary much when the molecule concentration changes.
The Process of Exciton-Polariton Funneling
Following UV irradiation, E-4-dimethylamino-4′cyanostilbene (trans-DCS) conducts intramolecular charge transfer (ICT), which is a highly conjugated donor-acceptor arrangement.
Because of the excited state's strong conjugation and ICT nature, the structural dipole moment increases dramatically, giving this organic molecule a massive Stokes shift and significant two-photon activity. Because of these two qualities, trans-DCS might be useful in a variety of applications, including two-photon stimulated fluorescence microscopy.
Donor-acceptor dyadic structures like ICT active molecular isomers are widely used in nonlinear molecular optics and organic optoelectronics.
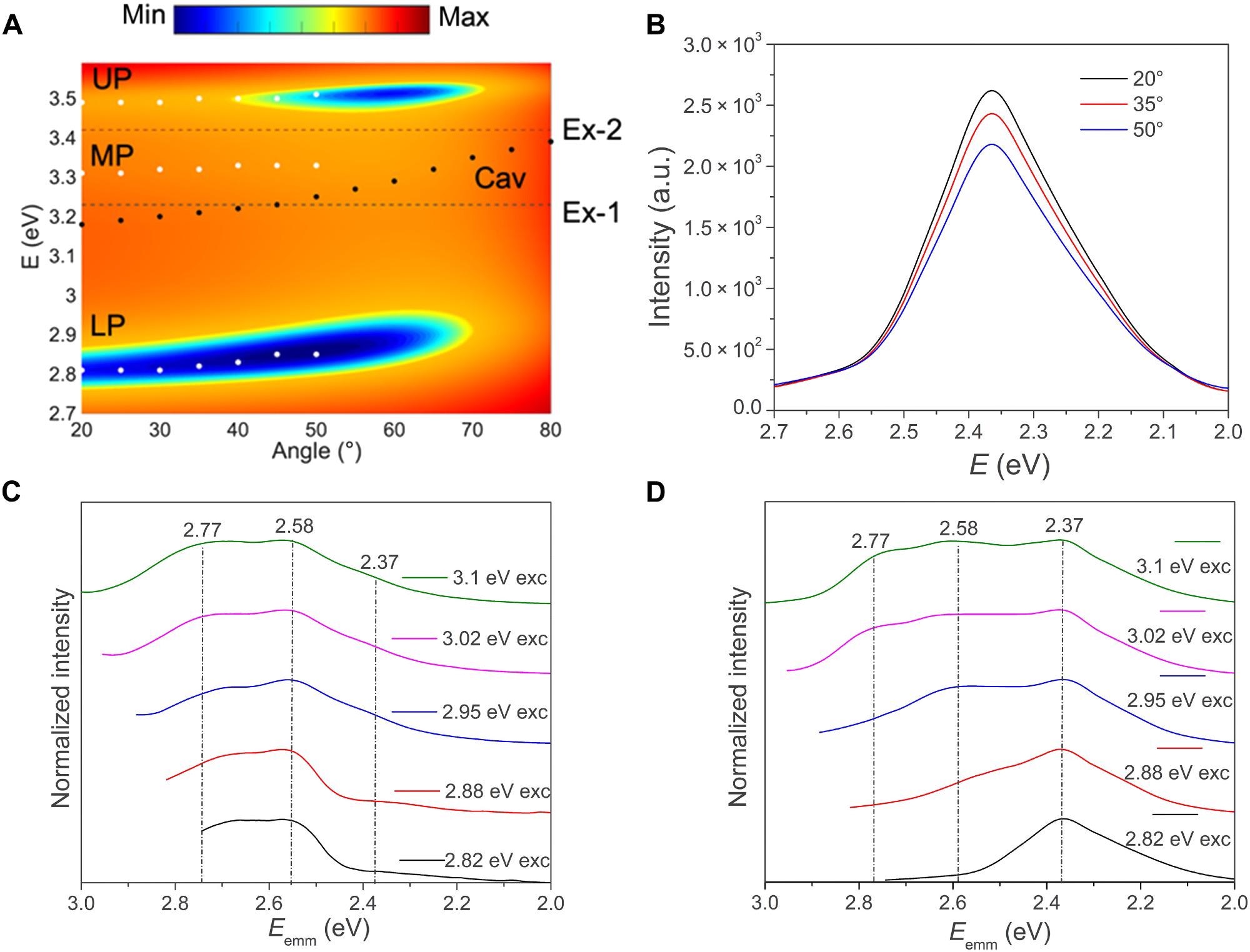
Optical characterization and excitation pump–dependent PL of the cavity sample. (A) Angle-dependent reflection (TM polarization, 20° to 80°) data for a strongly coupled DCS cavity sample (white filled circles denote the measured polariton dispersion, while black circles and dashed lines indicate the bare cavity and exciton dispersion data, overlaid on the simulation results, respectively), (B) Angle-resolved PL from the strongly coupled cavity sample for excitation energy of ~2.8 eV and normalized excitation energy-dependent PL spectra comparison for the (C) bare molecular films and (D) strongly coupled cavities of DCS (c = 1.2 wt%). Image Credit: Sataphaty, S., et al., Science Advances
The choice and purity of isomer emission, on the other hand, determine the overall effectiveness of the usage. Whenever the Rabi splitting is raised and the optical pumping is close to the LP resonance, the designed funnel process becomes more and more effective, culminating in full spectral weight modulation and significant brightening of pure TICS exciton emission.
With the reduction of LP energy levels, the implementation of TICS excited-state emission in the strong coupling regime gains significantly in intensity.
After photo-excitation of the P-trans and NP-trans electronic state of DCS, the planar excited state (PES) and TICS isomers are generated, respectively, in terms of their excitons.
The DFT calculated values predict a negligible energy barrier in absorption energy sources for the two scenarios, with the NP-trans relatively increased in energy than the P-trans, which explains why, in comparison to the PL spectrum, the absorption spectrum does not show extra functionality associated with the NP-trans isomer as molecular concentration was increased.
The rise is due to more NP-trans molecules, which means more TICSs are available (note that TICS emissions grow with concentrations in DCS bare molecular films, but it is still much lower than that from PESs). TICS emission is always more than that of PESs, regardless of the excitation energy utilized.
Hence, the majority of the increase must originate from somewhere else. As a consequence, our findings reveal that when the system reaches the strong coupling regime, a new relaxation route opens, allowing for efficient excitation transmission from the LP model to the TICS isomer, which then becomes the primary method when the resonance frequency drops below a certain threshold.
Reference
Sataphaty, S., et al. (2021). Selective isomer emission via funneling of exciton polaritons. Science Advances, DOI: 10.1126/sciadv.abj0997 https://www.science.org/doi/10.1126/sciadv.abj0997
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.