The use of environmentally friendly carbonaceous materials as catalysts for organic reactions has gained major attention in the past decades. Against this backdrop, graphene oxide (GO) exhibits exclusive structures, different surface functional groups, and superior properties. This is further explored in the journal Chinese Chemical Letters.
 Single-layer graphene oxide. Study: Recent advances in graphene oxide catalyzed organic transformations. Image Credit: Daniel Ramirez-Gonzalez/Shutterstock.com
Single-layer graphene oxide. Study: Recent advances in graphene oxide catalyzed organic transformations. Image Credit: Daniel Ramirez-Gonzalez/Shutterstock.com
The properties of GO include a large surface area in a single or few layers, acidic properties due to carboxyl groups on the edges, oxidative properties assisted by hydroxyl or epoxy on the basal plane, conduction band (CB) energy, and valence band (VB) energy.
Such properties make GO or modified GO potential materials in organic synthesis, energy, biology, and other fields such as nanocarriers for efficiently impairing tumor mitochondria and heterogeneous carbon catalyst for biomass conversion by controllable depolymerization of lignin.
This study reviews the significance of GO catalysis and the widespread applications of GO in organic synthesis over the past decade. The study also includes the application of native GO for (1) oxidative reactions (including oxydehydrogenation and oxidative coupling reactions), (2) functional group transformations, (3) Friedel-Crafts reactions, (4) condensation reactions, and so on.
Oxidative Reactions
Oxidative reaction, especially oxydehydrogenation, is one of the most common transformations in organic synthesis. This study is the first one to use GO as a co-catalyst to facilitate the synthesis of small molecular organic compounds under visible light irradiation.
Moreover, the GO catalyst can be reused five times without significant loss in catalytic activity under optimized conditions. It was found that GO not only acts as an acidic catalyst but also as an oxidative catalyst.
A series of control experiments have been conducted to investigate the reaction pathway of GO-mediated cross-dehydrogenative coupling of oxindoles. The results indicated that carboxyl groups (-COOH) and oxygenated groups, and quinone-type functionalities in GO, played synergistic roles in this transformation.
GO can be recycled up to six times without significant loss of catalytic activity (from 91% to 79%). A plausible mechanism of this reaction showed that GO could oxidize alcohols to give the corresponding aldehyde with the aid of a strong base, generating reduced graphene oxide (r-GO), which was re-oxidized by O2.
Functional Group Transformations
2,5-Diformylfuran (DFF) can be used as an important building block for the synthesis of furan-containing polymers and materials. Control experiments suggested that the synergistic effect of carboxyl groups (-COOH) and unpaired electrons at GO is responsible for the high selectivity and conversion of the reaction for DFF synthesis.
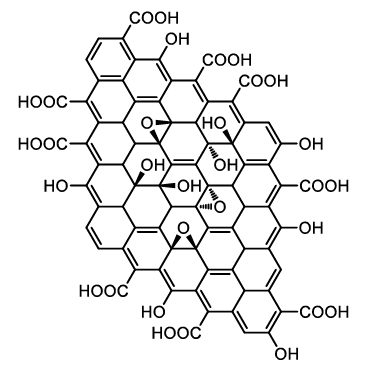
Structure of Graphene Oxide. Image Credit: Image Credit: Gao, et al., 2021.
Anilines and phenols can be brominated using molecular bromine in water catalyzed by GO at room temperature. These transformations possessed high selectivity for the tribromoanilines and tri-bromophenols. Moreover, when N-bromosuccinimide (NBS) is a brominating reagent, GO displayed excellent recyclability without any loss in catalytic activity after several cycles.
GO-catalyzed iodination of arenes and ketones with iodine in CH3NO2 as solvent was demonstrated. This iodination protocol not only obtained mono-iodination of arenes and ketones with good to excellent yields, regardless of the electronic effects but also achieved di-iodination of arenes and ketones with moderate to good yields by increasing the amounts of iodine to 4 equivalent.
Friedel-Crafts Reactions
An atom economic reaction of arenes and styrene or arylmethanols was promoted by GO to obtain diarylalkanes. This protocol was carried out under metal-free and additive-free conditions with good functional group tolerance and excellent regioselectivity to deliver the corresponding products in high yields.
Moreover, GO also displayed efficient catalytic activity for the synthesis of 3,3'-bisindolylmethane derivatives from indole derivatives and ethers at room temperature and air atmosphere.
Condensation Reactions
A simple, efficient methodology was developed to produce α-aminophosphonates and 3,4- dihydropyrimidin-2-ones using a three-component one-pot reaction, which was catalyzed by low loading of GO in a solvent-free environment under ultrasonication. This pathway showed that GO can be used as a mild, non-toxic, and sustainable catalyst for the multicomponent coupling reaction.
The GO-mediated direct 2-alkylation of 1,3-dicarbonyl compounds was developed with high reactivity, excellent regioselectivity using abundant olefins and alcohols as alkylating agents. Moreover, the results of FT-IR and XPS indicated that polar functional groups had negligible changes on the GO.
Conclusion
Graphene oxide (GO) is an economical carbon material that can be used as a metal-free carbocatalyst and efficient acidic catalyst for different organic reactions. This study summarized the latest advances of native GO-promoted organic reactions, such as oxidative coupling reactions, functional group transformations, oxidative halogenation, condensation reactions, etc.
In all reported systems, GO could be recycled and reused many times. But the use of GO as a light absorber and heterogeneous photocatalyst in visible-light-induced organic reactions is still uncommon. The use of graphene oxide as a heterogeneous photocatalyst in photocatalytic organic transformations could gain more interest in the future.
Journal Reference:
Gao, F., et al. (2021) Recent advances in graphene oxide catalyzed organic transformations. Chinese Chemical Letters. https://www.sciencedirect.com/science/article/pii/S1001841721009219?via%3Dihub