Why Titanium-Niobium-Oxide is a Suitable Anode Material for Lithium-ion Batteries?
Carbon-based materials such as graphite are the most commonly used anode material in commercial lithium-ion batteries (LiBs). However, it possesses drawbacks such as lower electrochemical potential, lower theoretical capacity, easy formation of a solid electrolyte interface (SEI) film due to the consumption of electrolytes, and safety concerns associated with its low melting temperature. Titanium-niobium-oxide, TiNb2O7 (TNO) can be a better alternative to graphite-based anode materials owing to its high theoretical capacity of 388 mAh g−1, high discharge voltage of 1.65 V, and long life span. However, the electrochemical performance of TNO is affected by its low ion diffusivity and poor conductivity.
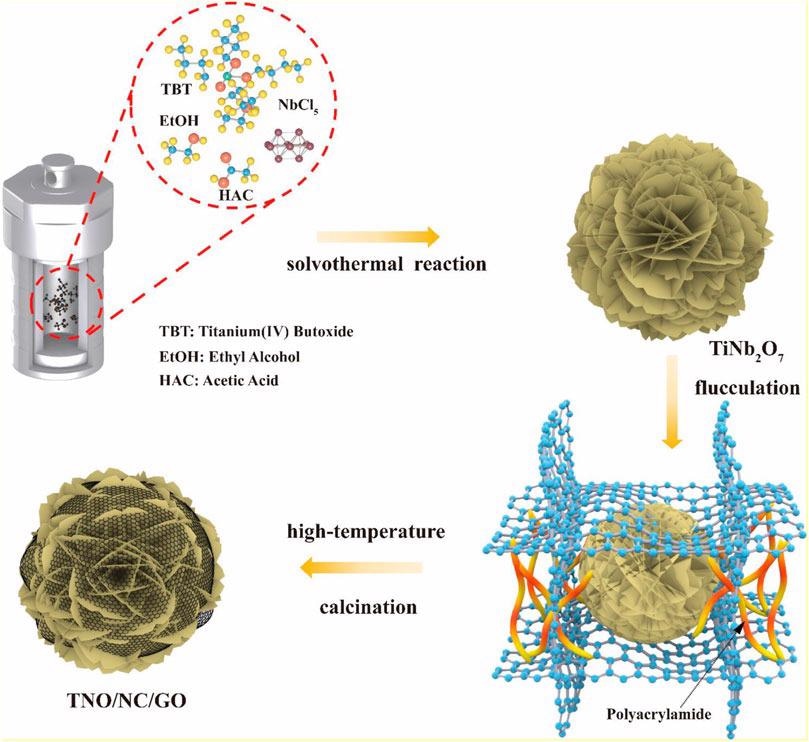
Schematic process of the formation of TNO/NC/GO composite. Image Credit: Hu, L et al., Frontiers in Energy Research
Carbon coating can be an effective way to resolve the poor conductivity of TNO, but it is difficult to achieve a homogeneous TNO/graphene composite due to the lack of sufficient electrostatic driving force and contact surface area. Another approach is to shorten the lithium-ion diffusion path by reducing the particle sizes or constructing different structures.
About the Study
The researchers synthesized a hierarchical TNO by controlling the degree of hydrolysis of esterification reaction between acid and alcohol. The flower-shaped layered structure of fabricated hierarchical TNO facilitates a short and efficient Li+ ion migration path and superior rate capability.
The growth of the flower-like hierarchical nanostructure is possible due to two factors. Firstly, the alcoholysis of niobium chloride (NbCl5) in the presence of ethanol inhibits hydrolysis during esterification and thus prevents unexpected precipitation and facilitates uniform nucleation and growth of complex structures. Secondly, polyacrylamide (PAM) as a binder provides strong adhesion between TNO and graphene oxide (GO) interface that overcomes the lack of sufficient electrostatic forces.
High purity TNO is synthesized through a solvothermal process, in which NbCl5 and titanium (IV) butoxide (Ti(OC4H9)4) are added into a solution of ethanol and acetic acid followed by heating and precipitation. GO powder is synthesized through modified Hummer’s process.
Finally, pure TNO powder is dispersed into graphene followed by drop-by-drop addition of PAM into the above suspension to initiate the cross-linking reaction. The high-temperature annealing/calcination of the suspension removed the solvent and reduced the graphene oxide to form the TNO-NC-GO composite. A working electrode is fabricated by mixing TNO-NC-GO composite, super P carbon black, and polyvinylidene fluoride (PVDF) and coating onto the copper foil.
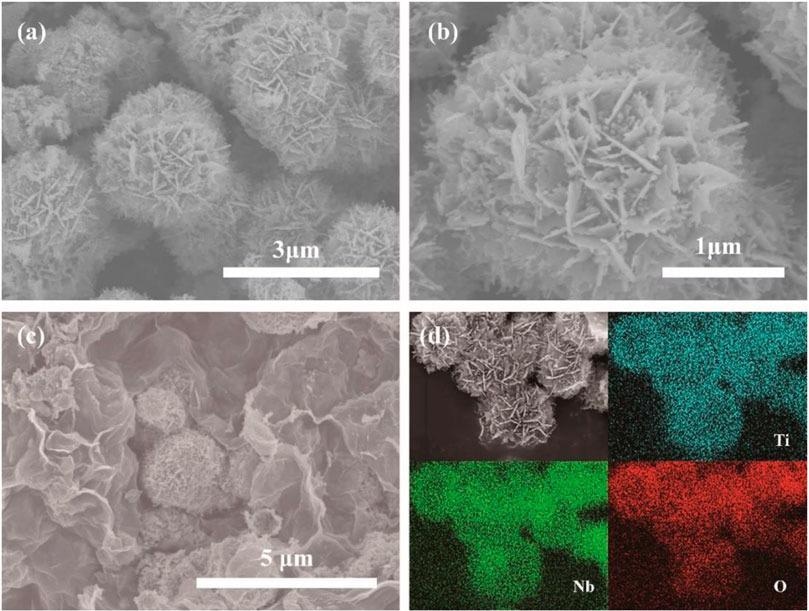
(A) SEM image of pristine TNO, (B) enlarged magnification SEM image of pristine TNO, (C) SEM image of TNO/NC/GO, (D) EDS mapping of different elements. Image Credit: Hu, L et al., Frontiers in Energy Research
Observations
The X-ray diffraction (XRD) patterns confirm the formation of pure TNO and the ideal reduction of GO into graphene after the annealing process.They also indicate the presence of disordered amorphous carbon in ordered graphene, which is the ion conductivity of the composite.
Scanning electron microscopy (SEM) shows the uniform distribution of TNO hierarchical nanoflower structure, which prevents the agglomeration of the TNO microstructure, forms a better dispersion on graphene, and provides low capacity loss during cycling. From the cyclic voltammetry (CV) curves, it is evident that TNO-NC-GO composite displays a larger response current than that of pure TNO and TNO-NC, the higher electrochemical response is due to an increase in conduction.
Moreover, the presence of GO enhanced the pseudocapacitive Li+ charge storage across the entire potential range. the galvanostatic discharge and charge curves of the TNO-NC-GO as anodes at different current densities show a capacity retention ratio of 85% after 200 cycles, which is almost double that of pure TNO. The volume expansion of the discharged composite anode is 8.9% after 1500 cycles, which is much lesser than silicon-based anodes (300%).
The interphase contact resistance obtained from Nyquist plots indicates that TNO-NC-GO composite has lower surface charge transfer resistance of 97Ω compared to that of pure TNO (66Ω) and TNO-NC (69 Ω), indicating a much faster mass transport due to the effect of carbon coating.
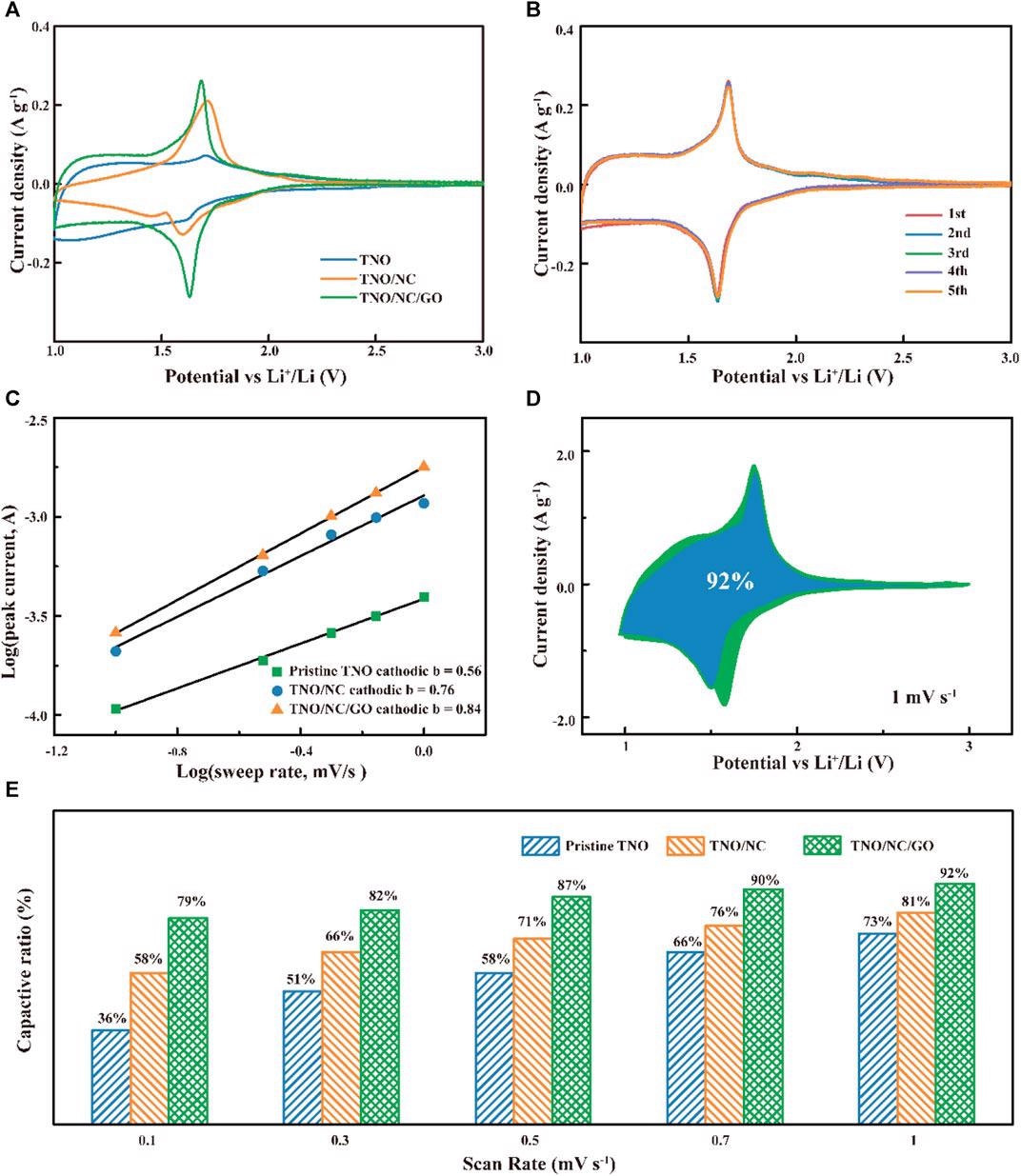
(A) Comparison of cyclic voltammetry curves at a scan rate of 0.1 mV s−1; (B) CV of TNO/NC/GO electrodes for LIBs. (C) the b-value obtained from the relationship between the peak current and sweep rate; (D) CV curves of TNO/NC/ GO with separation between total current and capacitive currents at 1 mV s−1; (E) capacitive contributions at different sweep rates for different samples. Image Credit: Hu, L et al., Frontiers in Energy Research
Conclusions
In this work, researchers prepared a novel flower-shaped hierarchical TNO nanostructure via an esterification reaction. The TNO-NC-GO composite is constructed via a simple cross-linked reaction by using PAM as a flocculant.
The new composite anode exhibits enhanced storage performance to that of pure TNO under identical conditions. With a high specific capacity of 264.2 mAh g−1 and excellent cyclability with extremely less capacity loss after 1500 cycles, it proves to be an ideal anode material that can replace graphite in future LiB manufacturing.
Reference
Hu, L., Yang, X., Chen, Y., Wang, L, Li, J., Tang, Y., and Zhang, H., Facile Construction of Hierarchical TiNb2O7/rGO Nanoflower With Robust Charge Storage Properties for Li Ion Batteries via an Esterification Reaction. Frontier Energy Research, 9, 794527. https://www.frontiersin.org/articles/10.3389/fenrg.2021.794527/full
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.