Humans rely on oxygen for survival. They metabolize around 200 grams of oxygen daily, and if a person goes without oxygen for longer than 10 minutes, it can cause irreversible damage and even death. Oxygen depletion is still an underestimated cause of fatalities worldwide and is one of the leading causes of death worldwide.
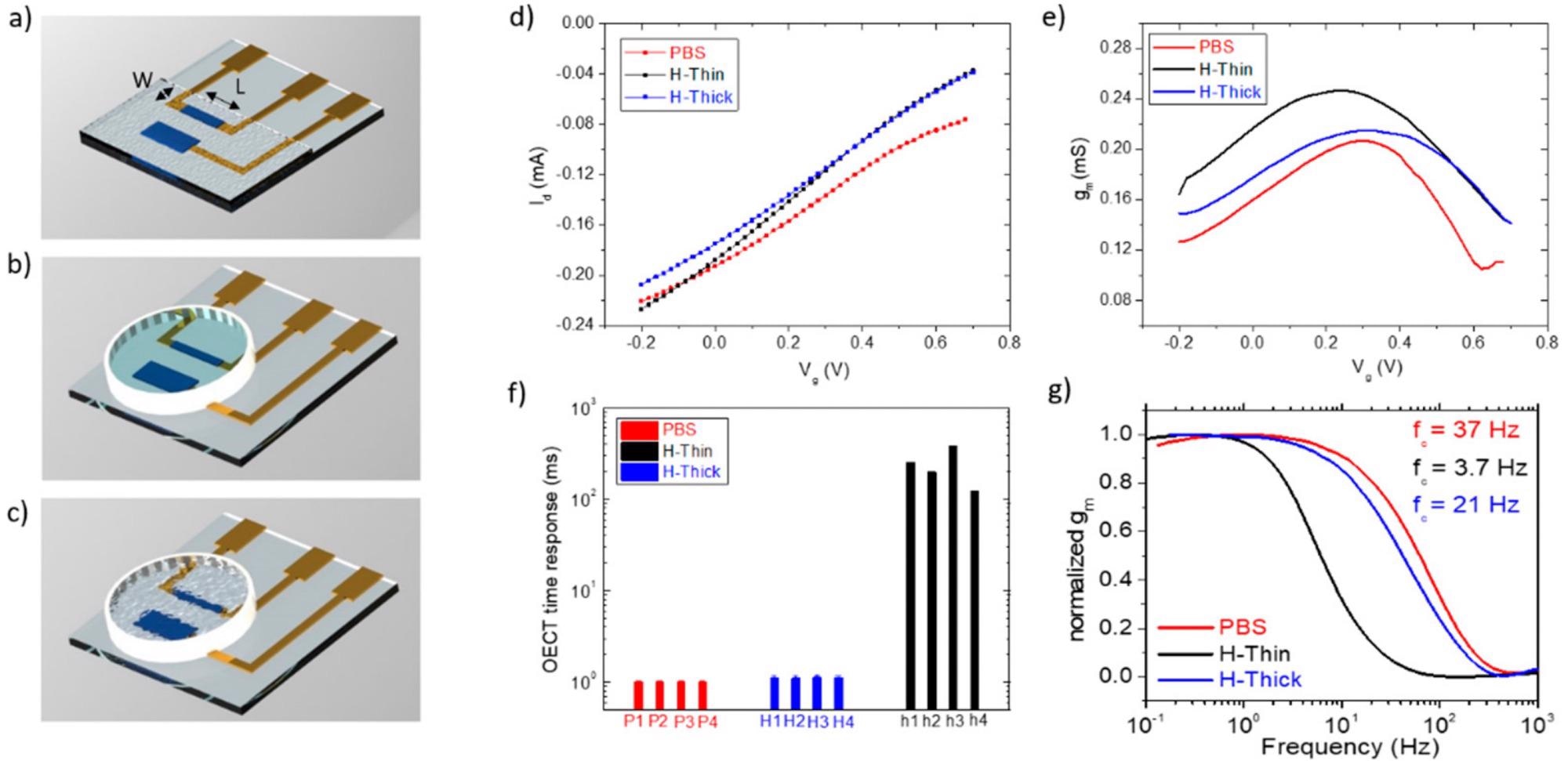
OECT configuration and electrical characterization. Rendering of the OECT devices on glass used with the thin hydrogel coating (a) and the thick hydrogel (b) or PBS electrolytes (c); (d) transfer; (e) transconductance; (f) time response to a voltage pulse on the gate of the OECT having PBS (red); the thin hydrogel (blue), or the thick one (black), as an electrolyte for gating. (g) Transconductance versus frequency measurements for OECTs having PBS (red); the thin hydrogel (blue); or the thick one (black); as electrolyte for gating, with relative cutoff frequencies (corresponding to the—3 dB points) extracted at 37, 3.7 and 21 Hz, respectively. Image Credit: Decataldo, F et al., Polymers
Oxygen can become depleted for several reasons. Leaks of inert gases such as nitrogen and helium, stored ripening fruits, combustion, microorganisms, rusting metals, and organic decomposition can all cause low levels of oxygen, especially in confined spaces. Recognizing the scale of the issue, many governmental organizations require the installation of oxygen gas sensors to monitor the levels of oxygen in confined spaces and warn workers when it is dangerously low.
Oxygen Sensors
Many commercial oxygen sensors are available on the market today which take advantage of various detection methods. Sensors developed include variants that continuously monitor the presence of gases and can be transported by workers where they are needed. However, these solutions can be expensive, bulky, and cannot be used as standard equipment by workers.
To tackle these issues, wearable sensors have been widely proposed and researched. Wearable sensors require not only superior sensing performance, but also superior mechanical performance and flexibility as they must be able to deal with significant deformation from everyday use, as well as being lightweight, biocompatible, and low cost. This class of sensors can be directly integrated into a worker’s overalls or personal protective equipment to continuously monitor oxygen and gas levels in the worker’s immediate environment.
Several reviews and studies have highlighted the heightened interest in this technology, with innovative state-of-the-art advances and implementations being reported. Amongst wearable sensors, conducting polymer-based devices have been investigated recently due to their low cost, stretchability, and flexibility.
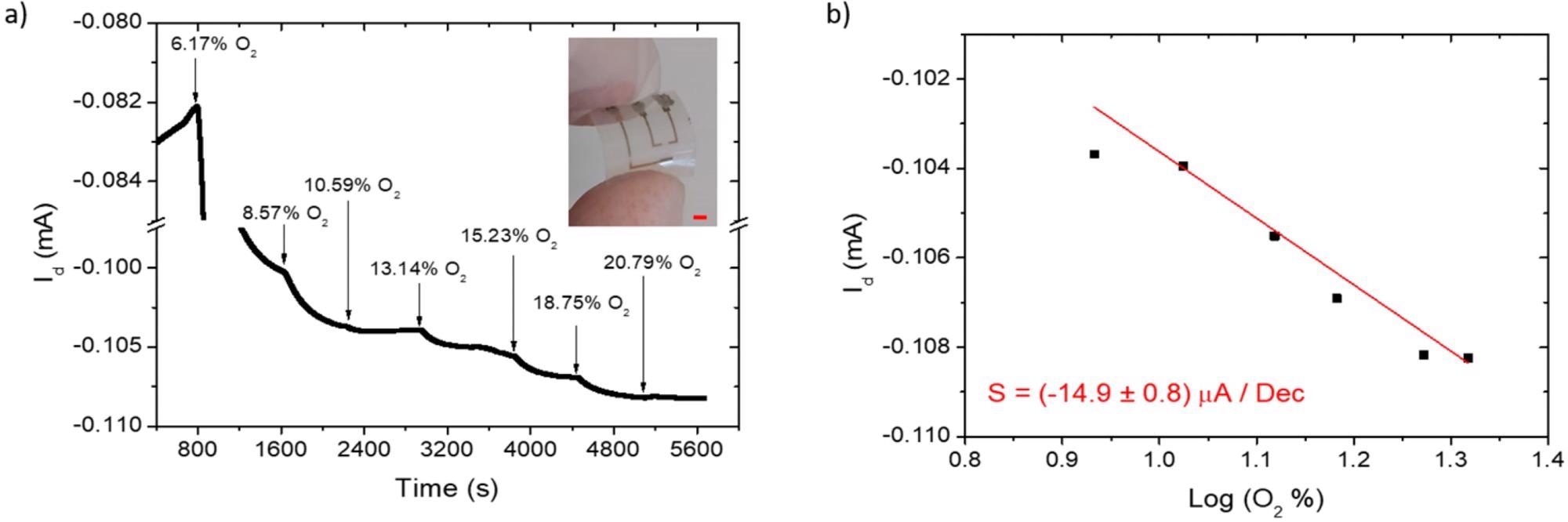
H-Thin OECT on PEN substrates for oxygen sensing: Id(t) measurement (a); sensor calibration curve (b); for oxygen addition (black arrows) in the sealed cell, starting from a nitrogen-saturated environment (0% of O2). The device picture is reported in the inset of (a); with a red scale bar of 2 mm. Image Credit: Decataldo, F et al., Polymers
The Study
The authors of the study have expanded on their previous work on PEDOT:PSS organic electrochemical transistors. In their previous work, they used these novel transistors to monitor the concentration of dissolved oxygen in cell mediums for biological applications. Organic electrochemical transistors have seen wide application as chemical sensing devices as they consume little power and provide easy functionalization and inherent signal filtering and amplification.
In the present study, the authors have created a cost-effective and reliable oxygen gas sensor which can monitor the levels of atmospheric oxygen using their previously developed system, with an important modification. The device in the previous work contained an electrolyte solution, but as this is not suitable for the detection of atmospheric oxygen as it does not reach a rapid equilibrium with the surrounding environment, the researchers replaced it with a conducting agarose hydrogel.
The proof-of-concept devices were fabricated using glass/plastic substrates, metallic contacts, deposited chromium and gold, a spin-coated PEDOT:PSS solution, and coated with the agarose hydrogel. Devices with a thin hydrogel layer were further refined using dip-coating. Finally, the organic electrochemical transistors were incorporated into transparent 125 µm thick polyethylene napthalate films to create flexible, wearable oxygen gas sensors.
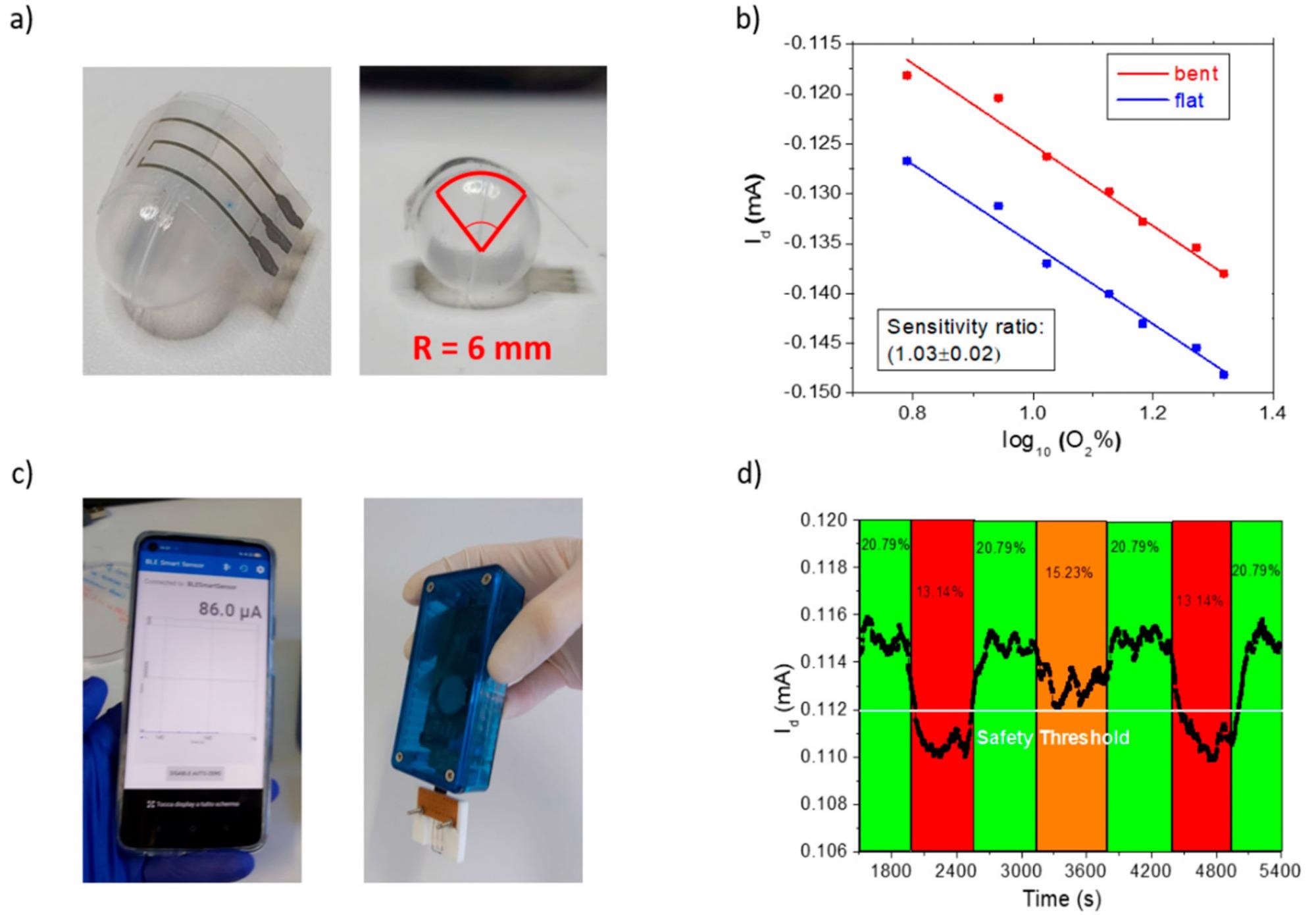
Bending test: (a) OECT patterned on PEN foil bent with a radius of 6 mm to mimic a real-life application; (b) OECT sensing the oxygen additions in the complete range from 6% to 21% in the flat (blue) and bent (red) configuration: (c) interfacing the sensor with a portable, handheld, battery-powered electronic readout from Elements srl company, wirelessly transmitting the current signal to a smartphone application; (d) warning test on a bent sample connected to the Elements system, showing the current response upon oxygen level variations (oxygen percentages are reported in black in the corresponding colored interval time). A safety threshold has been highlighted in white at 15% O2 to ensure operator’s protection before oxygen depletion could cause breathing fatigue or fainting. Image Credit: Decataldo, F et al., Polymers
Results of the Study
The results of the author’s experiments and analysis demonstrated that the proposed device was effective at sensing low levels of atmospheric oxygen. The thin, ~30 µm hydrogel coating additionally improved ease-of-use for device wearing and handling and can be noninvasively added to worker overalls and personal protective equipment.
The device’s current was linearly correlated to the oxygen percentage’s logarithm, making it highly sensitive. Additionally, the results were reproducible and repeatable, meaning the novel hydrogel-coated PEDOT:PP organic electrochemical transistor is reliable. Repeated bending tests demonstrated no loss of efficiency, meaning that the sensors were suitable for applications where flexibility is a key requirement.
Furthermore, the authors demonstrated that the sensors can be easily interfaced with a portable reader. This allows for real-time sensing of oxygen levels and the ability to set different detection thresholds to further improve worker safety. With this study, the authors have demonstrated a proof-of-concept flexible hydrogel-based sensing device that is low cost and reliable and can vastly improve worker safety in confined environments. This paves the way for integrated, wearable, and protective “wear-and-forget” oxygen sensing devices.
Further Reading
Decataldo, F et al. (2022) Oxygen Gas Sensing Using a Hydrogel-Based Organic Electrochemical Transistor for Work Safety Applications [online] Polymers 14(5) 1022 | mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/5/1022
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.