Oil spills can occur during the exploration, exploitation, transportation, and processing of petroleum, either as a result of accidents or for technical reasons, resulting in major environmental consequences. Aerogels, as excellent absorbents, may absorb oil in a water-oil mixture efficiently and selectively.
Many aerogels, however, still have issues such as limited mechanical strength, oil absorption capacity, and recyclability. Researchers are becoming interested in cellulose as a low-cost natural material because of its global availability, biocompatibility, and flexibility. It's been utilized to make ultralight absorbent aerogel materials for a long time. The majority of cellulose-based aerogels, on the other hand, have a brittle mechanical performance.
Many functional nanomaterials, such as polyimide, poly(N-isopropylacrylamide), and polyvinyl alcohol (PVA), have been employed as reinforcing materials along with nanocellulose to enhance the mechanical properties of aerogels to overcome the shortcomings of cellulose aerogels. PVA with long polymer chains aids in the production of high-density bonding of hydrogen with nanocellulose, which can improve the aerogel's mechanical qualities by generating strong contacts.
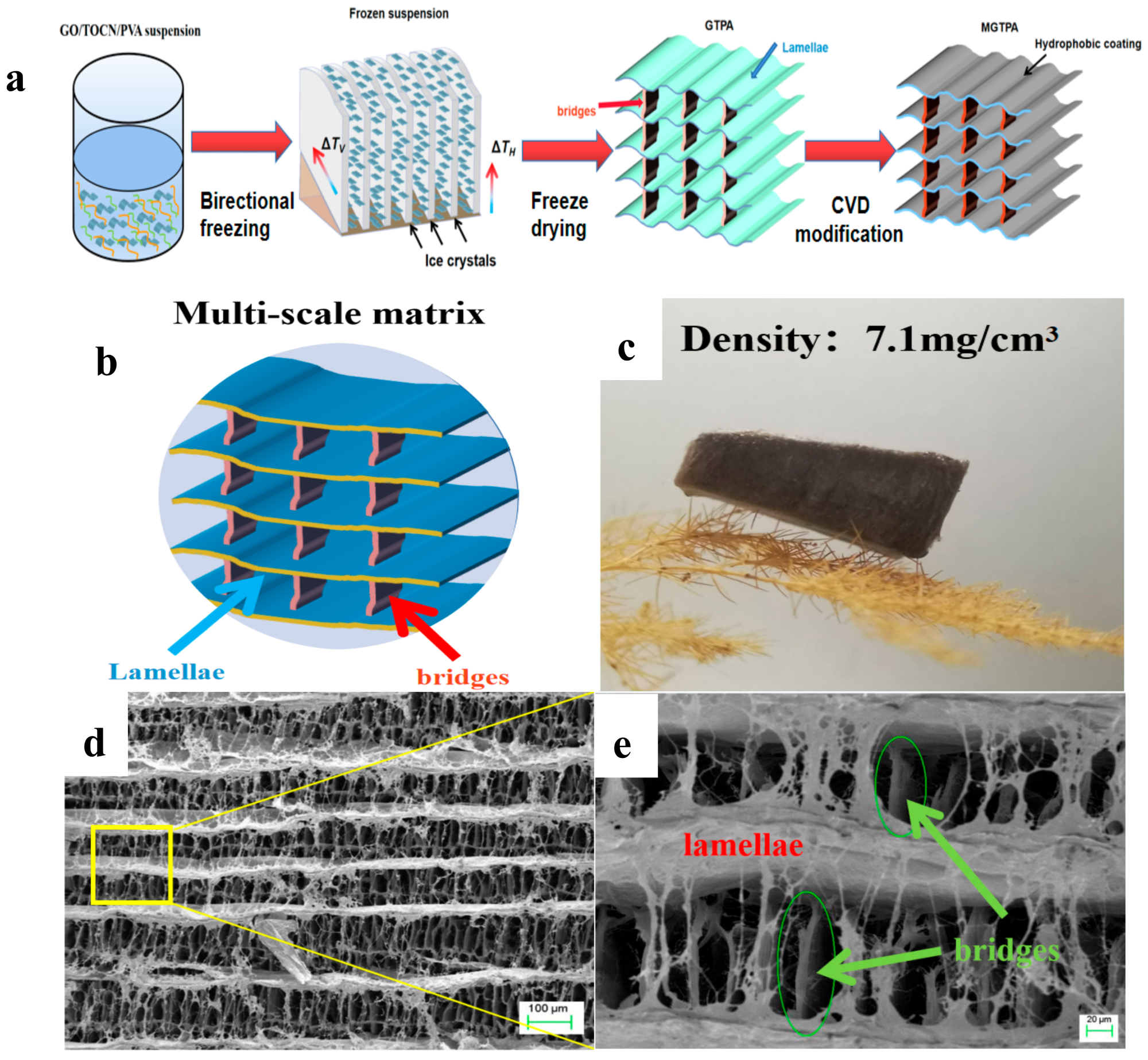
(a) Schematic illustration of the fabrication process of b-MGTPA. (b) Schematic of the b-MGTPA with a mineral bridge structure. (c) Photograph of an ultralight b-MGTPA located on a dogstail grass. (d,e) SEM images showing the architecture of the biomimetic aerogel at different magnifications. Image Credit: Feng, P et al., Polymers
About the Study
In the present study, the authors presented the fabrication of highly reusable graphene oxide (GO)/TEMPO-oxidized cellulose nanofiber (TOCN)/polyvinyl alcohol (PVA) aerogels with excellent mechanical properties and an architecture similar to that of Thalia dealbata stems through a three-step process of bidirectional freezing, freeze-drying, and chemical vapor deposition (CVD) modification. Through CVD modification, long carbon chains were grafted onto the surface of GTPA which resulted in the modified GTPA (MGTPA) hydrophobicity (WCA = 134).
A highly compressive graphene/PVA/cellulose nanofiber aerogel was created using a combination of bidirectional freezing and the inclusion of reinforcing elements. Then, to gain hydrophobicity, CVD modification was carried out utilizing simple sialylation.
Scanning electron microscopy images were used to examine the microstructures of the three different types of aerogels. The sessile drop method was used to measure the contact angle at room temperature. While the quality and size of the aerogel were measured using an electronic and digital caliper, the compressive properties of MGTPA were examined using an Instron 5565 universal material tester to study the mechanical properties of aerogel. The reusability of b-MGTPA was assessed by distilling and squeezing the oil, and the removal rate was calculated after 10 absorption–desorption cycles.
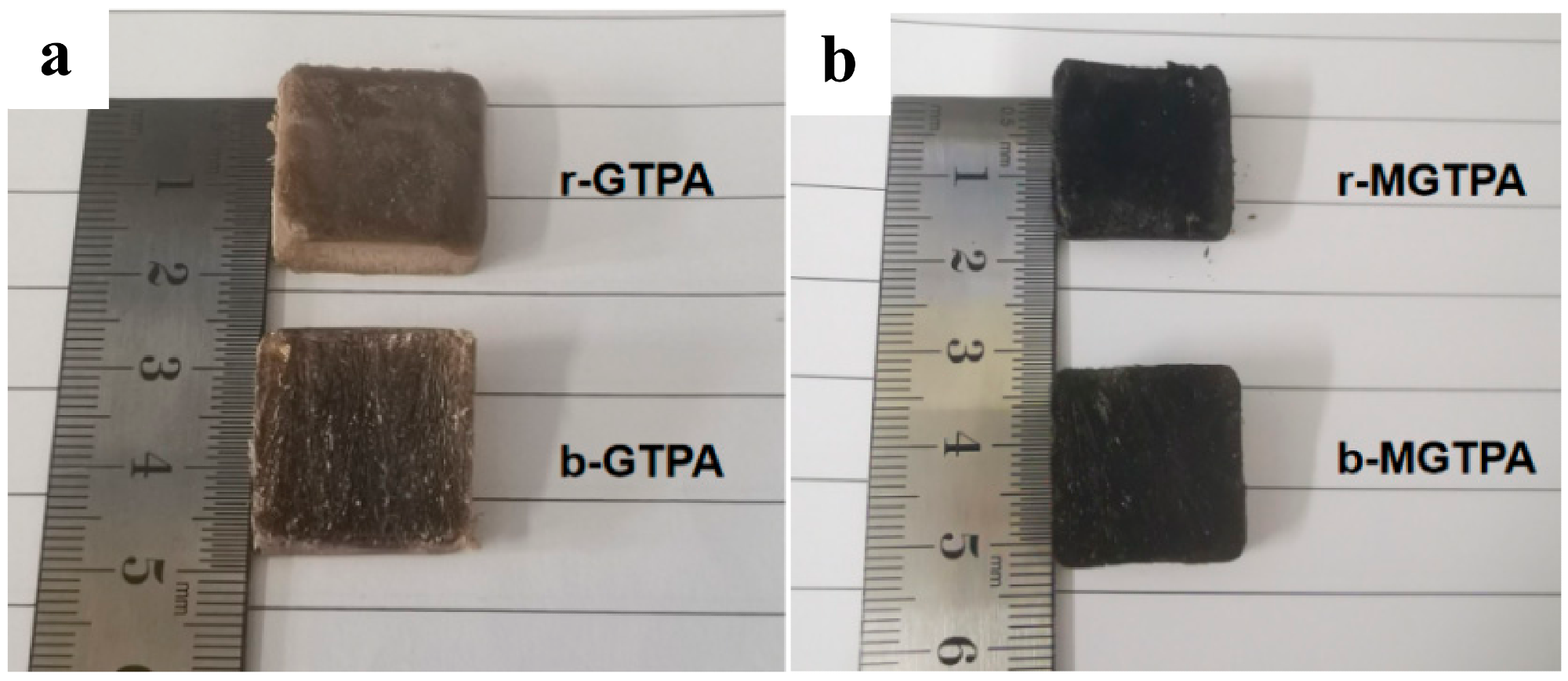
(a) Photograph of r-GTPA and b-GTPA. (b) Photograph of r-MGTPA and b-MGTPA. Image Credit: Feng, P et al., Polymers
Observations
After the 10th cycle test, the biomimetic structure was able to retain more than 92% of its initial saturation absorption capacity. The residual oil rate of the b-MGTPA in the cycle was around 15–20%. At 1 s and 180 s, the contact angles of the modified aerogel to water were 136 and 134.8, respectively, which indicated surface hydrophobicity. It was also observed that when the strain was less than 45%, the stress increased linearly with strain due to the bridge's elastic bending.
After being compressed for 100 cycles, MG20TPA had the highest initial strength of 98.4%, as well as the highest stress-retention rate after 100 and 300 cycles, with very little plastic deformation. Due to its unique structure of lamellar layers with bridges, b-MGTPA had an excellent recovery rate of 98.4% and maximum stress of 99.3% from 60% strain after 100 cycles. Furthermore, b-MGTPA had a high absorption capacity of 75–151 times its weight.
After 10 cycles, the adsorption capacity of b-MGTPA dropped by less than 10% during squeezing and less than 2% during distillation, showing a high degree of reusability. Simple mechanical extrusion recovered about 80–85% of the absorbed oil.
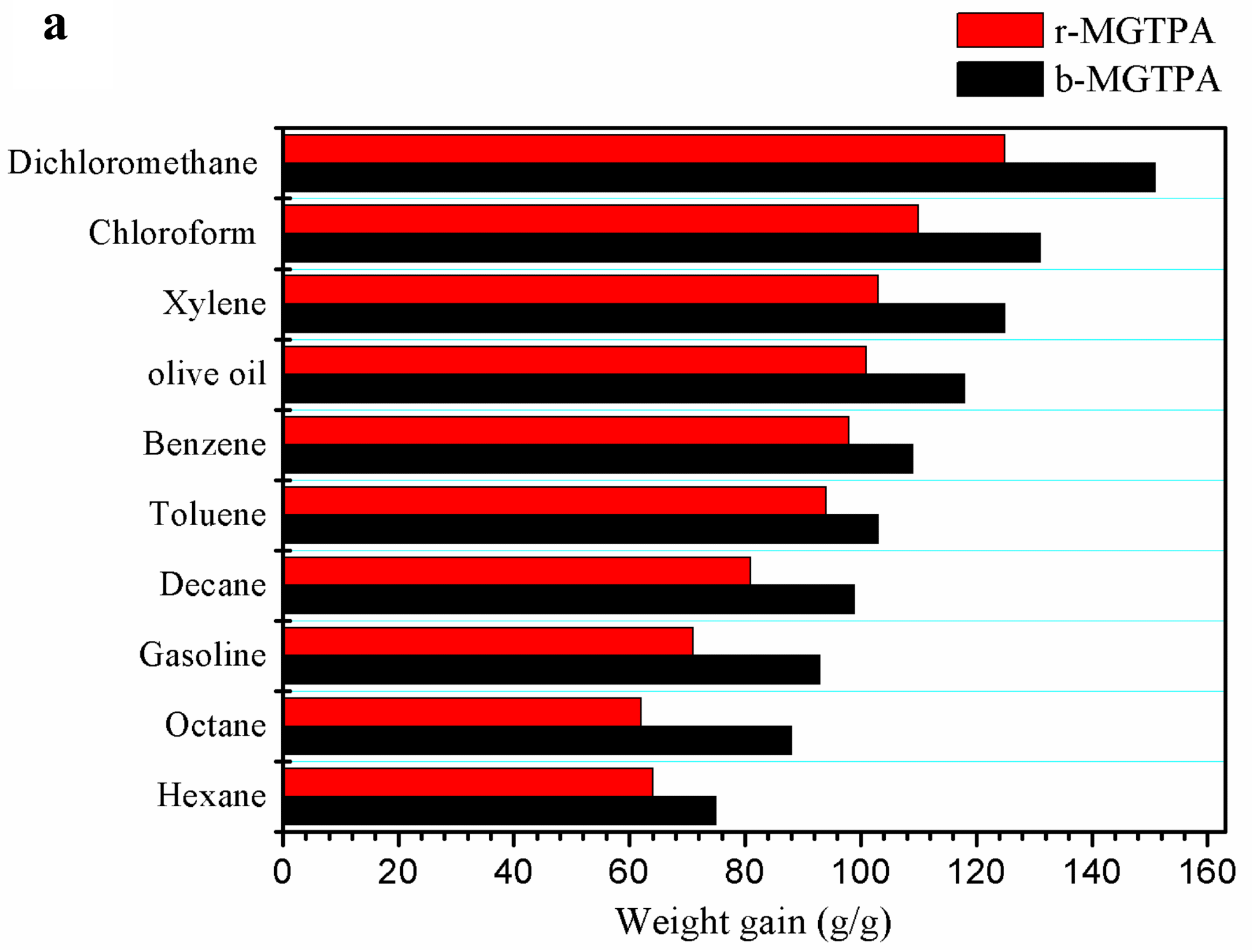
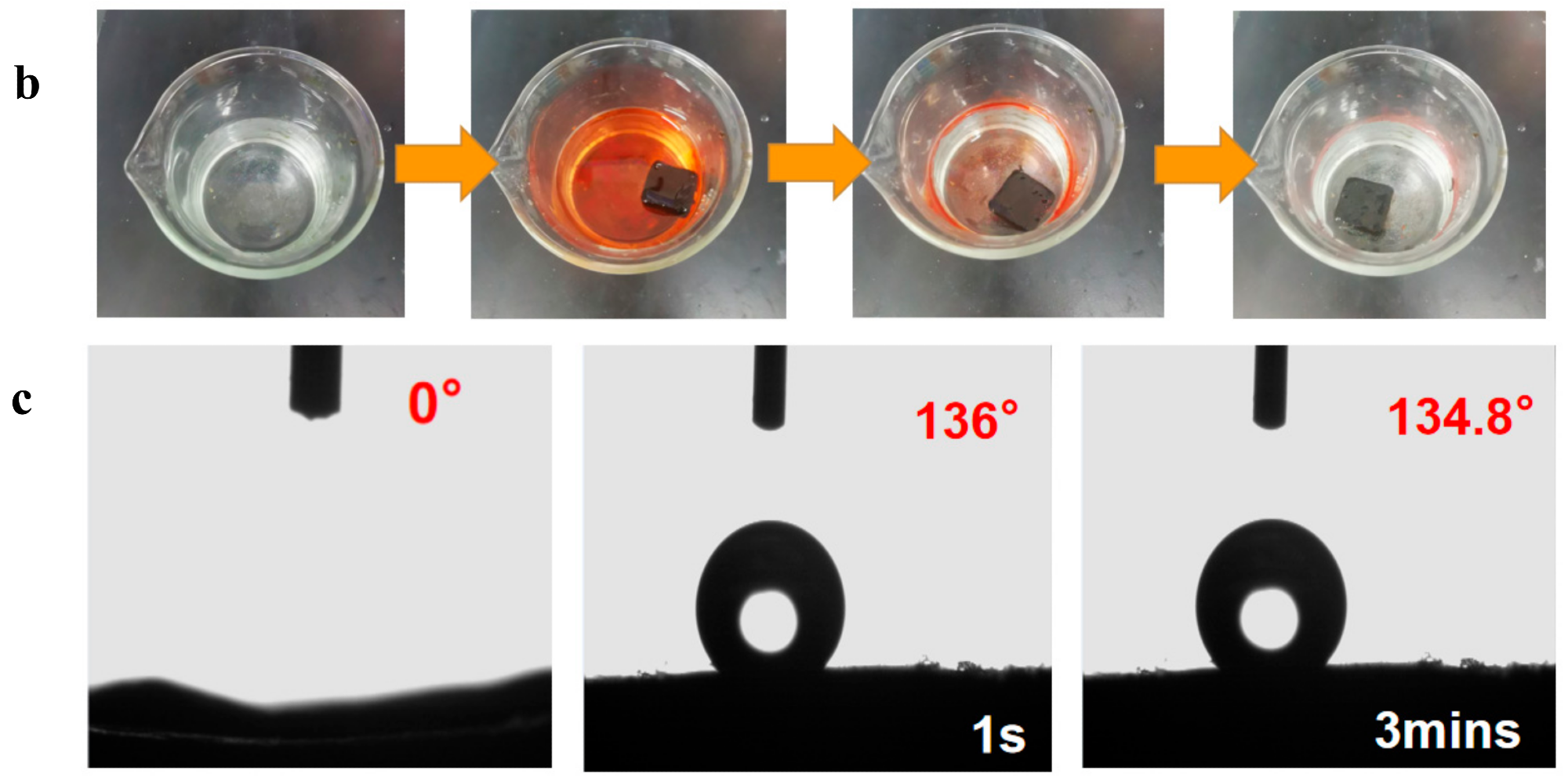
(a) Absorption capacity of d-MG20TPA and b-MG20TPA for various solvents. (b) Demonstration of selective oil (dyed red with Sudan III) absorption atop water using b-MGTPA. (c) Water contact angle of the b-GTPA and b-MGTPA taken at 1 s and 3 min. Image Credit: Feng, P et al., Polymers
Conclusions
In conclusion, this study demonstrated the development of a biomimetic, compressive, and hydrophobic graphene/PVA/cellulose nanofiber aerogel having unique three-dimensional (3D) linked lamellar layers with bridges by using bidirectional freezing. b-MGTPA had outstanding compressible qualities due to the synergistic effects of the three components and the unique biomimetic structure. For several types of organic solvents, b-MGTPA demonstrated a high adsorption capability. Furthermore, because of its high compressibility, b-MGTPA could recover absorbed oil quickly and efficiently using simple mechanical squeezing, and it had excellent reusable stability.
Furthermore, even after 10 extrusion or distillation cycles, b-MGTPA demonstrated exceptional reusability, retaining a high absorption capacity. Due to its unique biomimetic structure, the b-MGTPA retrieved more than 90% oil by mechanical squeezing.
Overall, the authors believe that because of its hydrophobic properties, low cost, easy preparation, and consistent performance, b-MGTPA has a lot of potential in cleaning water spills and as organic solvents.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Feng, P., Wang, X., Yang, J., Biomimetic, Highly Reusable and Hydrophobic Graphene/Polyvinyl Alcohol/Cellulose Nanofiber Aerogels as Oil-Removing Absorbents. Polymers 14(6), 1077 (2022). https://www.mdpi.com/2073-4360/14/6/1077