Previous literature shows CO2- or amine-based reactive carbon solutions as methane sources in electrochemical systems. However, these systems involve additional steps like thermal extraction of CO2 and purification of products.
Copper (Cu) preferably promotes methane production under acidic conditions. However, the pH at the cathode surface is generally greater than 12 due to OH−. Although this alkalinity suppresses undesirable hydrogen production (HER), it also suppresses the production of required protons for methane production.

Scanning electron microscopy images of the fresh copper foam (top) and the etched copper foam (bottom) which was used to mediate bicarbonate electrolysis. Image Credit: Lees, E. W. et al., ACS Energy Letters
About the Study
In the present study, the authors designed and constructed an electrochemical reactor to convert aqueous KHCO3 into methane. The bipolar membrane (BPM) present in the membrane electrode assembly (MEA) provides protons to porous Cu cathode aiding in CO2 in situ generations from KHCO3 solution followed by its reduction into methane. Adding cationic surfactant in electrolyte reduced HER at the cathode. The methane yield was 10-fold higher than in previous reports.
Further, the authors developed a one-dimensional (1D) continuum model to understand the working mechanism of the constructed electrochemical reactor. The results revealed that reducing electrolyte alkalinity favors methane production over multicarbon products.
The Faradaic efficiency (FE) and methane yield were determined using gas chromatography. Proton nuclear magnetic resonance (1H NMR) was employed to quantify the CO2 reduction reaction (CO2RR) product and formate.
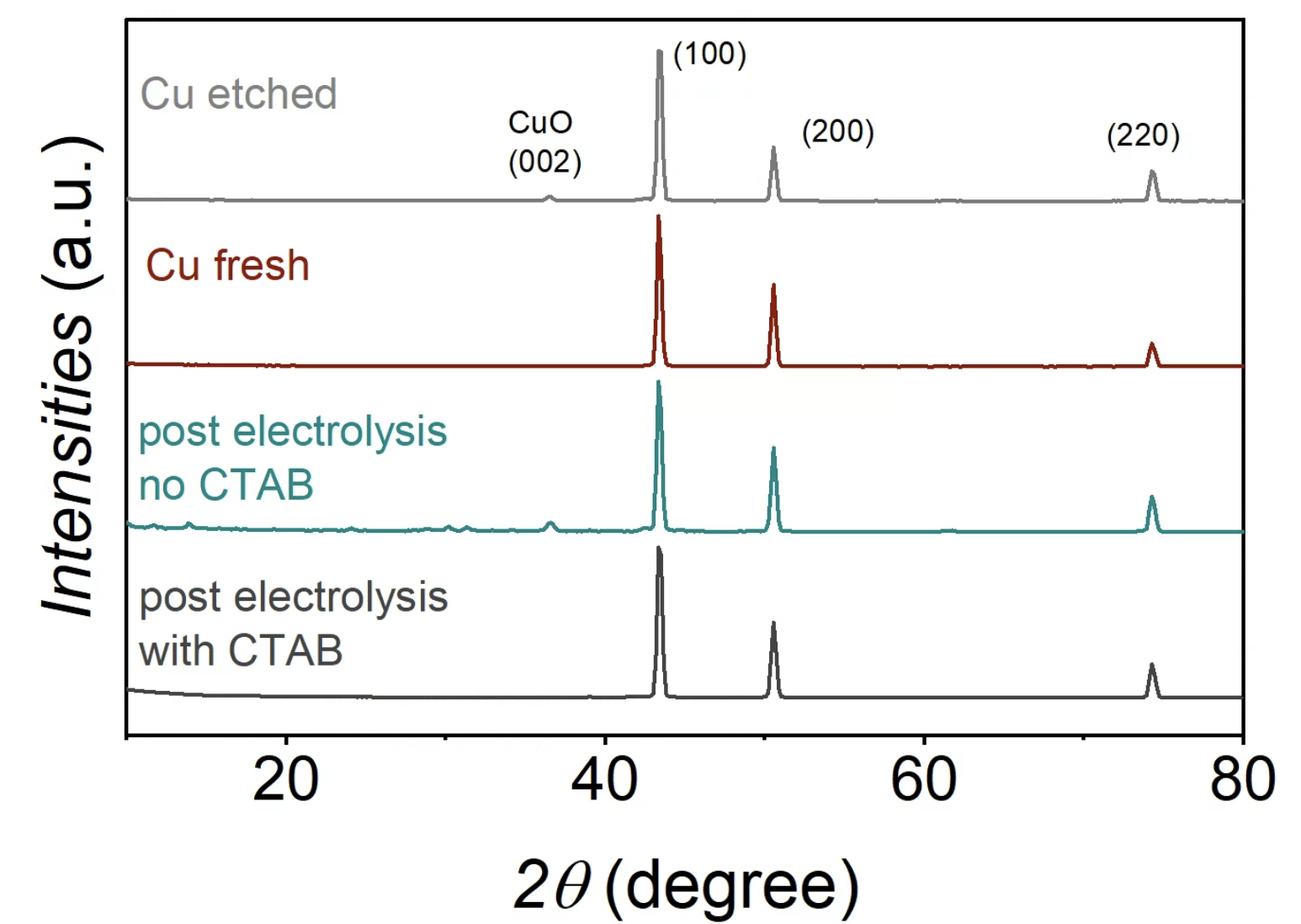
X-ray diffraction spectra for the fresh, etched, and post-electrolysis copper foam samples with and without CTAB. Image Credit: Lees, E. W. et al., ACS Energy Letters
Research Findings
The fabricated Cu cathode was analyzed using scanning electron microscopy (SEM), and the image showed an approximate pore size of 10 μm.
CTAB-assisted electrochemical reaction in a bicarbonate electrochemical reactor containing 3M KHCO3 solution at the cathode flow plate and 1M potassium hydroxide (KOH) at the anode flow plate using a range of cathode potentials and current densities resulted in only formate without methane, suggesting proton (H+) supply is crucial for methane formation from reactive carbon solutions. This electrolysis yielded a maximum partial current density for CO2RR products of 11 mA cm−2.
Further, X-ray diffraction (XRD) studies on the copper electrode revealed the presence of Cu (200), Cu (220), Cu (111), and cupric oxide (CuO) (002) facets before electrolysis. After electrolysis using CTAB, CuO (002) peak disappeared in XRD analysis. However, electrolysis conducted without CTAB showed this peak, suggesting that CTAB has a role in reducing the surface CuO layer on the copper electrode.
Interfacial methane formation between the catalyst layer and flow plate junction at a pH of 8.5 with an 8mM concentration of CO2 suggests that a thinner catalyst enables fast mass transport without compromising methane formation rates. Further, the ionomer maintained the local pH to enhance CO2 diffusion and improved methane formation rates. Thus, maintaining pH at cathode less than 12 enabled high methane production rates.
Bypassing the regeneration step (performed for pure CO2 production) in a bicarbonate electrochemical reactor resulted in less energy consumption than in a CO2-fed electrochemical reactor to reduce CO2 into methane. Also, minimizing unreacted CO2 resulted in high methane yield in the bicarbonate electrochemical reactor.
Carbon single-pass conversion was lower in the bicarbonate electrochemical reactor than in the CO2-fed electrochemical reactor. This reduction was due to bypassing downstream methane purification costs. Moreover, the methane formation in the bicarbonate electrochemical reactor is sourced from captured CO2 using a renewable energy source.
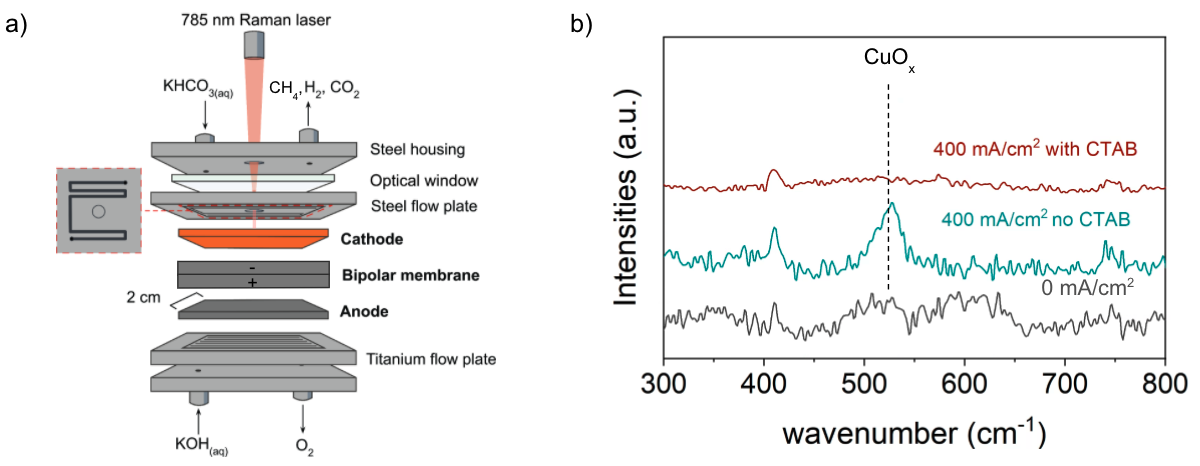
a) Schematic diagram of the bicarbonate electrochemical reactor cell used to perform in situ surface enhanced Raman spectroscopy. (b) In-situ Raman spectra of the copper cathode during electrolysis with (red) and without (green) 5 mM CTAB dissolved in 3 M KHCO3. Note that the spectra obtained at 0 mA cm-2 was unchanged by the presence of CTAB in the electrolyte. The characteristic Raman bands corresponding to vibrational modes of Cu oxides are indicated with dashed lines. Image Credit: Lees, E. W. et al., ACS Energy Letters
Conclusions
Methane production via electrochemical CO2 reactors involves purified CO2 gas as a carbon source. This process includes the thermal desorption of CO2 for upstream carbon capture, followed by downstream product purification.
The present work reported a novel method using a bicarbonate-based carbon solution for methane production. It is an energy-efficient alternative to CO2-fed electrochemical methane production. Using CTAB resulted in its adsorption to the Cu catalyst layer during electrolysis, enabling methane production at partial current densities of 120 ± 10 mA cm−2. The methane yield in this process was 34 ± 7%. Further, the results from the 1D continuum model at the cathode compartment revealed that H+ from BPM counteracted with the OH− generated at the cathode, resulting in the reduction of alkalinity and thus preferentially produced methane.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.