Global energy usage is predicted to rise by 50% by 2050, according to figures from the U.S. Energy Information Association. This huge increase in demand presents challenges for scientists and governments, as human activity has been intrinsically linked with global climate change and rising temperatures. To mitigate the effects of anthropogenic climate change, renewable and sustainable energy harvesting and storage technologies have been explored in the past few decades.
Electro-photochemical water splitting has emerged as a beneficial technology, where hydrogen is produced and can be used for energy storage due to its high energy density and total absence of carbon emissions. In this process, abundant, efficient, low-cost, and stable electrocatalysts are essential. To date, platinum has been commonly used as a catalyst for hydrogen evolution reactions due to its electrochemical inertness, high activity, and near-thermoneutral binding energy.
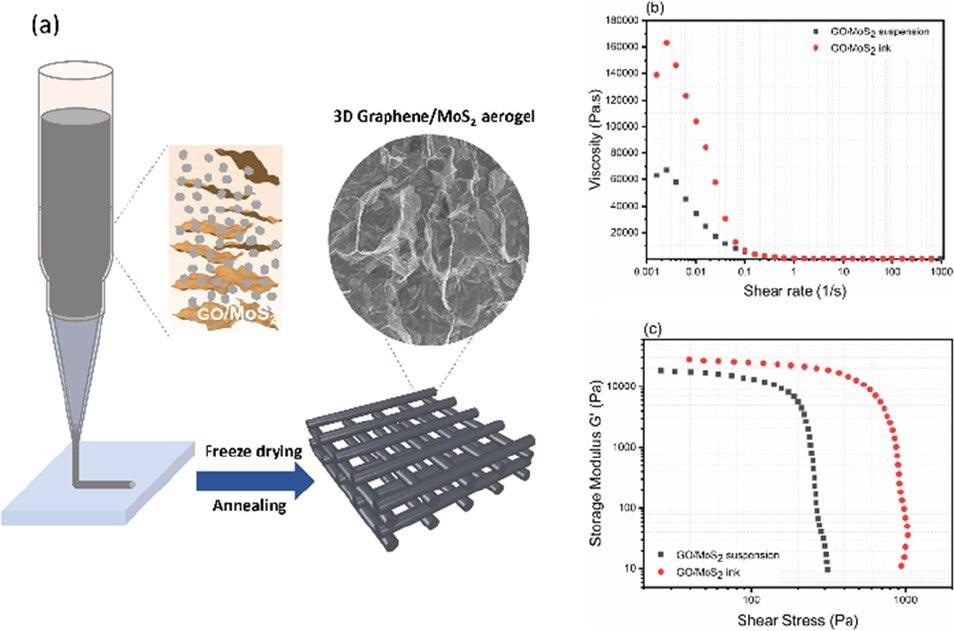
(a–c) DIW of GO/MoS2 with rheological studies. Image Credit: Chandrasekaran, S et al., ACS Materials Au
Layered Transition Metal Dichalcogenides: Next-generation Materials
Several low-cost, abundant, and stable materials with superior performance have been explored in recent years for use as electrocatalysts for hydrogen evolution reactions. Of these, layered transition metal dichalcogenides have been a focus of research in materials science. The most promising and widely studied of these is MoS2, with both experimental and theoretical studies confirming the presence of catalytically active edge sites in the material.
However, their commercial application is hindered by the stacking and aggregation of 2D sheets. This reduces the number of available active sites for hydrogen evolution reactions. Electrical conductivity in these materials is inhibited, making it incredibly difficult to design reliable electrodes. To overcome this issue, scientists incorporate conductive fillers to produce composite materials. Graphene is a common filler for this purpose.
Graphene aerogels contain 2D sheets in a three-dimensional porous carbon framework. Their high surface area enhances the flow of electrolytes, possesses high electrical conductivity, and facilitates their use as catalysts. Moreover, these materials can be combined with other materials to provide a supporting scaffold which increases their catalytic activity.
Studies have been performed on creating MoS2/graphene aerogels with improved surface area and enhanced hydrogen evolution catalytic activity. 3D MoS2/graphene aerogels 6-8 times larger electrochemically active areas have been reported in the current literature. These aerogels have significantly enhanced catalytic activity. Other studies have demonstrated nitrogen-doped graphene oxide-incorporated aerogels with low overpotentials.
Electrocatalytic Electrode Fabrication
Conventional 3D electrocatalytic electrode manufacture involves processes such as electrodeposition, solution casting, and thermal gelation. It is well-known that catalyst properties are influenced by structure and composition, and the manufacture of 3D catalysts with two-dimensional nanosheets could provide new opportunities in catalyst design and the improved performance of these commercially important materials.
In recent years, 3D printing, otherwise known as additive manufacturing, has provided significant benefits for numerous industries such as manufacturing and biomedicine. Structures can be directly printed from a variety of precursor materials in a low-cost, low-waste, and flexible process with unique design freedom compared to conventional methods. Fused deposition modeling, direct ink writing, and selective laser melting have been widely explored for printing electrodes in recent years.
Direct ink writing is an extrusion-based process that prints layer-by-layer structures from an “ink” of various functional materials from an extrusion nozzle. The freedom of materials used in direct ink writing makes it beneficial for the printing of 3D graphene/MoS2 aerogel-based electrocatalytic electrodes. The rheological properties of printed structures can be tailored using additives such as inorganic fillers and polymers.
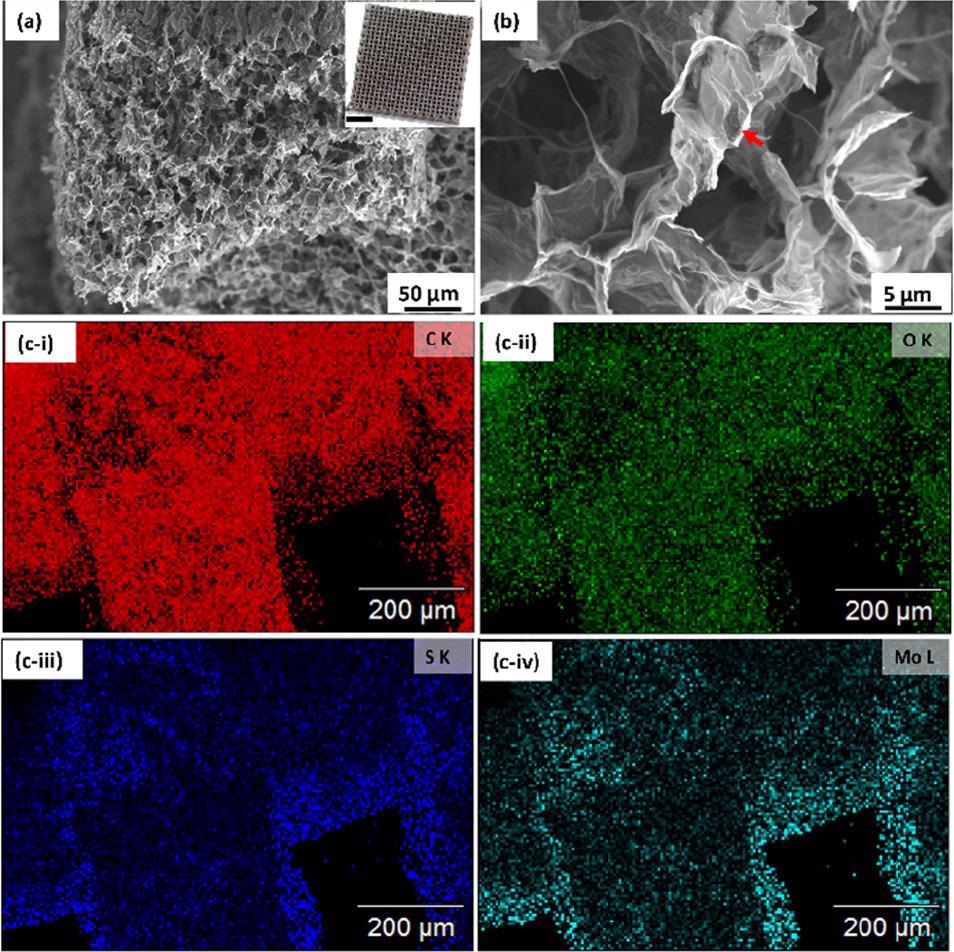
(a, b) Scanning electron micrographs of a 3D printed graphene/MoS2 aerogel (inset scale bar: 5 mm) and (c-i–iv) elemental image analysis of the GA/MoS2 aerogel. Image Credit: Chandrasekaran, S et al., ACS Materials Au
The Study
The study has reported the fabrication of a catalytic electrode by combining commercially available MoS2 powders and a graphene aerogel. The aerogel is extremely porous. By combining these materials and utilizing direct ink writing, the authors produced a hierarchical 3D catalysis framework. The printed electrocatalytic electrodes combine the advantages of both materials with the benefits of additive manufacturing.
A hybrid structured catalyst was produced using the novel process introduced in the research. MoS2 particles were highly concentrated in the electrocatalyst and were combined with graphene oxide sheets. Freeze drying and thermal annealing produced a structure with homogenously distributed MoS2 particles on the surface of an extremely porous graphene aerogel framework.
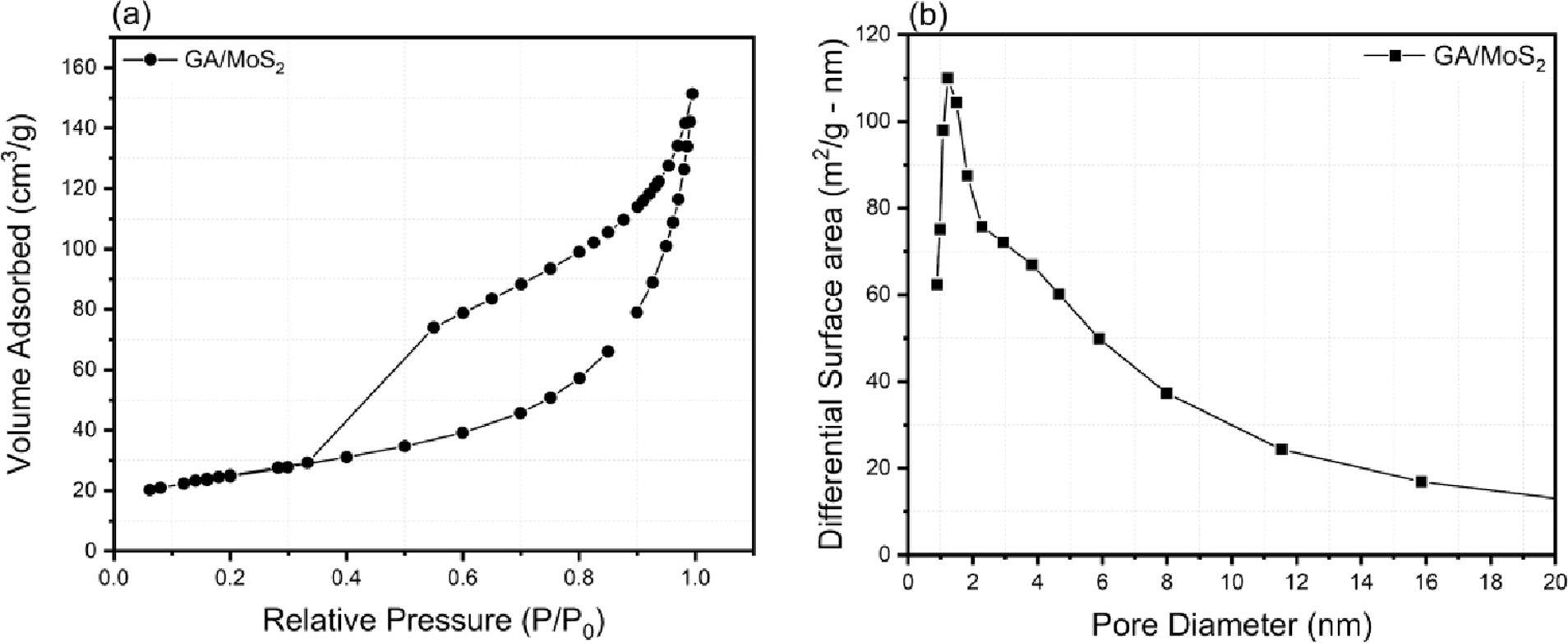
(a) Nitrogen adsorption–desorption isotherms and (b) BJH pore size distribution derived from the desorption isotherms of GA/MoS2. Image Credit: Chandrasekaran, S et al., ACS Materials Au
The composite electrode possessed a high surface area, and the hydrogen evolution performance at different currents was significantly enhanced in this material compared to pure graphene aerogel samples. The electrochemically active surface areas of the composite electrodes were not lost under high current operation, demonstrating the material’s robustness and mass transport control by 3D direct ink writing processes.
In short, the research has demonstrated the development of a robust and efficient electrocatalytic electrode and the value of additive manufacturing to produce next-generation electrochemical devices.
Further Reading
Chandrasekaran, S et al. (2022) Three-Dimensional Printed MoS2/Graphene Aerogel Electrodes for Hydrogen Evolution Reactions ACS Mater. Au [online] pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsmaterialsau.2c00014
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.