Natural photosynthesis precisely combines the storage and harvest of solar energy in chemical bonds through carbohydrate formation and water oxidation by dividing the overall redox reaction in photosystems II and I.
Water oxidation is a light-driven process, while the Calvin-Benson-Bassham cycle is a light-independent/dark process that converts electrons to carbohydrates as energy carriers. This decoupled electron storage and photoelectric conversion model can be mimicked artificially to develop off-grid solutions for intermittent and rapidly growing energy requirements.
Although significant progress has been made in storing electrochemical potential generated by photovoltaic cells in decentralized batteries, a suitable approach with a lower implementation cost and energy loss is required that combines energy storage, photo-conversion, and controlled energy release abilities in a single solar rechargeable system.
Materials enabling the conversion of solar energy and long-term storage of readily available chemical and electrical energy are critical for off-grid energy distribution. The application of carbon nitride- and graphene-based materials have been investigated for decoupled solar energy storage.
However, these materials cannot store energy for a long duration, which increases the challenge of realizing an efficient off-grid energy delivery system. Other challenges include the development of cost-efficient solar capacitors/batteries with high stabilities and efficiencies and lowering the processing and raw material costs.
MOFs are three-dimensional (3D) crystalline materials composed of coordinating organic linkers and metal-based nodes that collectively form networks with permanent porosity. MOFs are used as battery separators, water-splitting materials, and supercapacitors due to their combinatorial versatility.
These 3D matrices can be interfaced with discrete coordination complexes to achieve a higher level of sophistication in a material design inspired by the complex biological systems for applications in light-driven oxidations and solar fuel production.
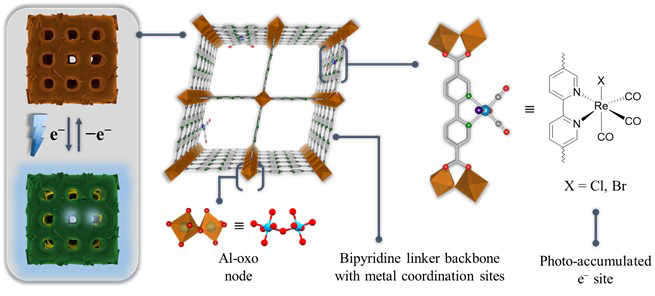
Schematic light-induced charge and dark discharge of the electron-accumulator and structural representation of its building blocks (Al-oxo nodes and 5,5’-dcbpy linkers). Vacant coordination sites are loaded with opto-electronically active Re sites. Red: O, Cyan: Al, Green: N, Grey: C, Blue: Re, Purple: X. Image Credit: Warnan, J et al., Advanced Materials
The Study
In this study, researchers investigated the feasibility of using a rhenium (Re) coordination complex interfaced with MOF-253 for decoupled energy storage and dark photocatalysis.
5,5’-dicarboxy-2,2’-bipyridine (5,5’-dcbpy) linkers and aluminum chloride (AlCl3) were used to synthesize pristine MOF-253 through the solvothermal method. Subsequently, the synthesized MOF-253 powders were soaked in acetonitrile (MeCN) solution of rhenium carbonyl complex (ReX(CO)5) (X=bromide, chloride) for 24 h at 65 oC to load the vacant 2,2’-bipyridine (bpy) sites, yielding hybrid ReBr(CO)3@MOF-253 (ReBr-253) and ReCl(CO)3@MOF-253 (ReCl-253), respectively.
Electron paramagnetic resonance (EPR), nitrogen adsorption measurements, powder X-ray diffraction (PXRD), solid-state ultraviolet-visible spectroscopy, attenuated total reflectance infrared (ATR-IR) spectroscopy, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy, and density functional theory (DFT) calculations were performed to characterize the synthesized samples.
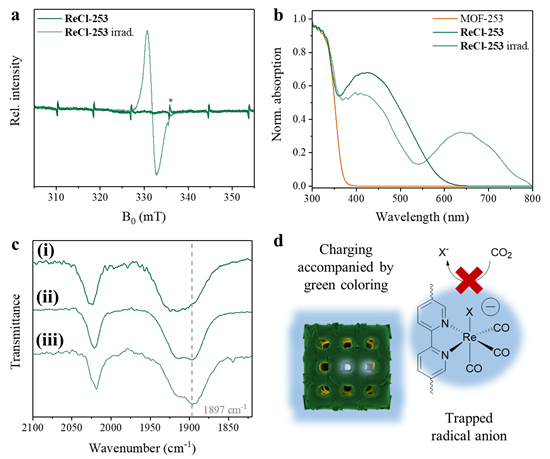
Characterization of ReCl-253’s properties after exposure to 450 nm irradiation in organic media with an electron donor. a Solid-state EPR spectra (g = 2.0034). *Mn2+ reference. b Solid-state UV-vis spectra. c ATR-IR spectra of (top to bottom) (i) as-synthesized ReCl-253, (ii) samples charged in MeCN with Ar atmosphere, and (iii) MeCN with BIH instead of TEOA. d Mechanistic rationale of charging, caused by radical anion formation and stabilization. Image Credit: Warnan, J et al., Advanced Materials
Observations
The Al node-based MOF-253 was effectively functionalized using a rhenium Lehn-type molecular complex, leading to the successful synthesis of ReBr-253 and ReCl-253 hybrid materials. The Re was distributed homogenously within the MOF matrix without affecting the MOF crystallinity. The particle size of ReCl-253 and MOF-253 samples was 1.05 ± 0.35 µm.
The pristine MOF-253 was stable up to 400 oC temperature. However, the stability decreased with the increase in Re loading, indicating the successful incorporation of ReX(CO)3 center into the MOF-253.
The synthesized hybrid materials accumulated and stored electric charges under visible light irradiation during the presence of an electron source. Electrons were accumulated consistently in both hybrid materials during the entire irradiation duration.
A maximum charge of 15 C∙gMOF−1 was accumulated in the ReCl-253 sample in 1.5 h of irradiation with 0.9% solar-to-output energy storage and conversion efficiency. Moreover, the charges were successfully stored for four weeks with negligible loss.
The maximum charge density reached 545 ± 34 C∙gMOF−1 when charged Re centers were loaded in all vacant bpy sites, which demonstrated the prospect of optimizing the hybrid material in the future to realize full electron-accumulation potential.
Additionally, the radical charging speed was modulated by altering the component concentrations and irradiation intensity to optimize the material for fast-charging applications.
The hybrid materials displayed a rechargeable electron delivery and storage behavior with only one% capacity loss per cycle and 11% loss after 10 cycles, unlike other Re-based hybrids and homogenous Re molecular catalysts that show catalytic activity. However, charge storage at 45 °C and above led to fast capacity loss.
Although the capacity loss in the hybrid ReCl-253 was higher compared to state-of-the-art photocapacitors that retain more than 95% capacity even after thousand cycles, the observation effectively demonstrated the feasibility of using MOF-based materials for energy storage. Moreover, the hybrid material displayed chemical stability, and its production can be scaled affordably and sustainably due to the use of low-cost AlCl3 and off-the-shelf linkers.
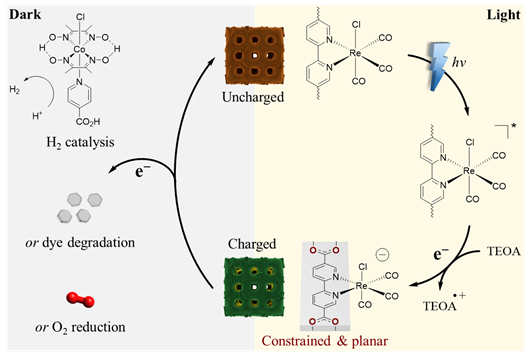
Working principle of decoupled light-induced charge and dark discharge of the ReCl-253 electron-accumulator. The constraining effect of MOF-253 on Re centers conditions electronic properties enabling long-lived electron storage. On-demand use of stored charges is possible by outer sphere electron transfer to (i) a cobaloxime-based H2 evolution catalyst, (ii) an organic dye effecting degradation, or (iii) O2 reduction. Image Credit: Warnan, J et al., Advanced Materials
This unique behavior of the hybrid materials was attributed to the MOF-253 confinement effect on the Re center molecular reactivity, which created conditioned electronic properties and unique complex geometry, leading to long-term electron storage. The electron density distribution induced the charge accumulation and electro-catalysis behaviors in hybrid samples.
The charge stored by the hybrid material was released on-demand and in the dark when it was coupled with an electrocatalyst to drive hydrogen production catalysis and redox reactions to realize decoupled, off-grid photoelectrochemical energy release and storage that mimicked the light and dark cycles of natural photosynthesis. This mechanism effectively tackled the issue associated with the intermittency of solar irradiation.
Rapid and selective use of stored electrons was realized through the transfer of outer sphere electrons to substrates that do not need a free coordination site on the Re central metal atom, including a cobaloxime-based hydrogen evolution catalyst and an organic dye affecting degradation.
Conclusion
To summarize, the findings of this study demonstrated the feasibility of using Re-functionalized 3D MOF for decoupled solar energy storage. This approach combined light harvesting, photoinduced-free electron generation, and storage into a single wireless material, eliminating the need for photoelectrochemical configurations.
However, more research is required to identify a suitable alternative to the precious metal Re-based site to increase practicability and reduce cost and new ways must be explored to couple stored electrons with electronic devices, including electrode fabrication, to ensure long-term stability and conductive contact in working conditions.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.