Image Credit: Tokyo University of Science
The team discovered a particular composition after conducting comprehensive characterization and electrochemical performance testing. This composition could pave the way for high-performance magnesium rechargeable batteries.
Given their extensive use in everything from portable devices to cellular base stations, lithium-ion batteries continue to be the industry leader in terms of overall performance for several applications. They do, however, have a few significant drawbacks that are difficult to overlook.
Lithium is fairly expensive, and the fact that it is being mined at a rapid rate makes matters worse. Additionally, a lithium-ion battery's energy density is insufficient to provide heavy machines and electric vehicles autonomy.
Scientists are searching for alternatives to batteries because of these worries and the fact that the batteries are extremely dangerous when punctured or heated.
Magnesium (Mg) is a viable option among the different elements being studied as effective energy carriers for rechargeable batteries. Mg is safe and abundant, but it also has the potential to produce batteries with greater capacity.
But first, a few issues need to be resolved. These include the Mg ion-provided low voltage window and the erratic cycling behavior seen in Mg battery materials.
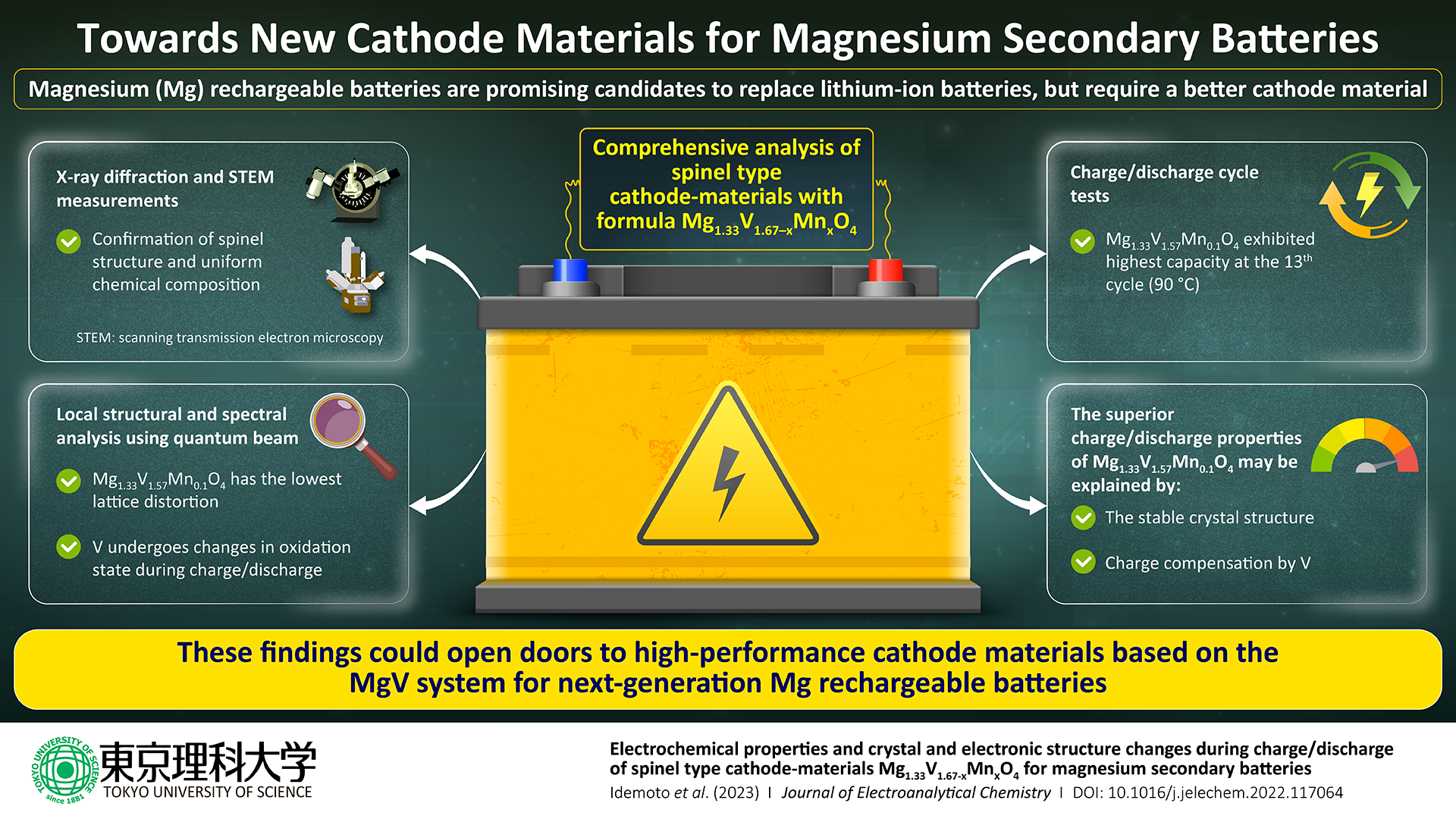
A research team from Tokyo University of Science, Japan, led by Vice President and Professor Yasushi Idemoto, has been searching for new cathode materials for Mg batteries to address these problems.
They have been focusing in particular on finding strategies to enhance the performance of cathode materials built on the MgV (V: vanadium) system.
Fortunately, they have finally discovered the path to success, as described in a recent study that was made accessible online on 8th December 2022 and published in Volume 928 of the Journal of Electroanalytical Chemistry on January 1st, 2023.
The Mg1.33V1.67O4 system was the main focus of the study, but some vanadium was replaced with manganese (Mn), yielding minerals with the formula Mg1.33V1.67–×Mn×O4, where × ranges from 0.1 to 0.4.
Although this system had a large theoretical capacity, further research was required to fully grasp its structure, cyclability, and cathode performance. As a result, the researchers used a wide range of conventional methods to characterize the synthesized cathode materials.
First, they used transmission electron microscopy, X-Ray diffraction, and absorption to examine the composition, crystal structure, electron distribution, and particle morphologies of Mg1.33V1.67×Mn×O4 compounds.
The investigations revealed that Mg1.33V1.67–×Mn×O4 has a very homogeneous spinel structure. To assess the battery performance of Mg1.33V1.67–×Mn×O4 using various electrolytes and examining the consequent charge/discharge behaviors at various temperatures, the researchers next carried out a series of electrochemical studies.
For certain cathode materials, notably Mg1.33V1.57Mn0.1O4, the research team found high discharge capacities, but they also found that these capacities fluctuated greatly depending on the cycle number. They examined the local structure in the vicinity of the vanadium atoms in the material to determine why.
It appears that the particularly stable crystal structure along with a large amount of charge compensation by vanadium leads to the superior charge-discharge properties we observed for Mg1.33V1.57Mn0.1O4. Taken together, our results indicate that Mg1.33V1.57Mn0.1O4 could be a good candidate cathode material for magnesium rechargeable batteries.
Yasushi Idemoto, Vice President and Professor, Tokyo University of Science
He drew a positive conclusion regarding the future while being content with the current findings and stated, “Through future research and development, magnesium batteries could surpass lithium-ion batteries thanks to the former's higher energy density.”
Indeed, replaced MgV systems could eventually result in the eagerly anticipated next-generation batteries. One can only hope that the much-awaited lithium replacement will materialize soon.
Japan’s JST ALCA-SPRING Grant Number JPMJAC1301 provided funding for this study.
Journal Reference:
Idemoto, Y., et al. (2023) Electrochemical properties and crystal and electronic structure changes during charge/discharge of spinel type cathode-materials Mg1.33V1.67–×Mn×O4 for magnesium secondary batteries. Journal of Electroanalytical Chemistry. doi:10.1016/j.jelechem.2022.117064