Image Credit: Angewandte Chemie.
The advancement of batteries for electric vehicles is primarily influenced by two key factors: power, which directly impacts the vehicle's range, and cost, a critical factor in competing with internal combustion engines.
The US Department of Energy is committed to expediting the shift from gasoline-powered vehicles to electric vehicles. It has established ambitious objectives to decrease production costs and enhance the energy density of batteries by 2030. It is important to note that conventional lithium-ion batteries cannot meet these targets.
A highly promising strategy for creating smaller, lighter, considerably more powerful, and safer batteries involves using solid-state cells with metallic lithium anodes instead of graphite.
In contrast to traditional lithium-ion batteries, which employ liquid organic electrolytes and a polymer film to divide the anodic and cathodic sections, solid-state batteries consist entirely of solid components. A slim ceramic layer serves the dual purpose of a solid electrolyte and separator.
This design is highly effective at preventing dangerous short circuits resulting from the formation of lithium dendrites and curbing thermal runaway. Furthermore, solid-state batteries do not contain easily flammable liquids.
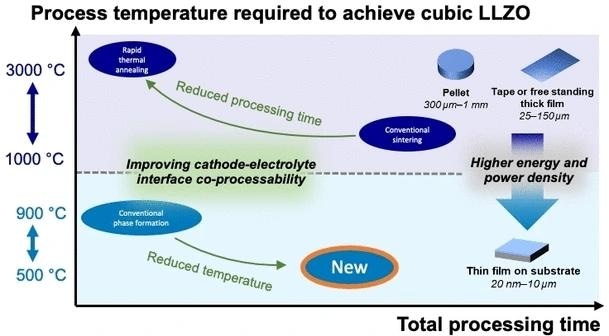
The garnet-type lithium oxide Li7La3Zr2O12−d (LLZO) is an example of a suitable ceramic electrolyte/separator for cells with high energy density. To transform the LLZO (lithium lanthanum zirconium oxide) material into the high lithium-conducting cubic crystalline phase, ensuring adequate density and a strong bond with the electrode, it currently requires sintering at temperatures exceeding 1050 °C.
However, temperatures surpassing 600 °C destabilize sustainable, low-cobalt, or cobalt-free cathode materials, simultaneously leading to increased production costs and energy consumption. New, cost-effective, and sustainable production methods are imperative.
A team led by Jennifer L. M. Rupp at MIT in Cambridge, USA, and TU Munich in Germany, has successfully devised a novel synthetic method. This innovative approach does not rely on a ceramic precursor compound but rather employs a liquid precursor that undergoes direct densification to create LLZO through a sequential decomposition synthesis.
To fine-tune the conditions for this synthetic process, Rupp and her research team scrutinized the multi-step phase transformation of LLZO, evolving from its amorphous state to the essential crystalline form (cLLZO). They employed several methods, including Raman spectroscopy and dynamic differential scanning calorimetry, and constructed a time-temperature-transformation diagram.
Leveraging the insights they acquired into the crystallization process, they devised a pathway in which dense, solid cLLZO is achieved through a 10-hour annealing process at a relatively low temperature of 500 °C, all without the need for sintering.
In future battery designs, this method will enable the integration of the solid LLZO electrolyte with sustainable cathodes, potentially eliminating the reliance on economically critical elements like cobalt.
Journal Reference:
Zhu, Y., et al. (2023). Time–Temperature–Transformation (TTT) Diagram of Battery‐Grade Li‐Garnet Electrolytes for Low‐Temperature Sustainable Synthesis. Angewandte Chemie International Edition. doi.org/10.1002/anie.202304581.