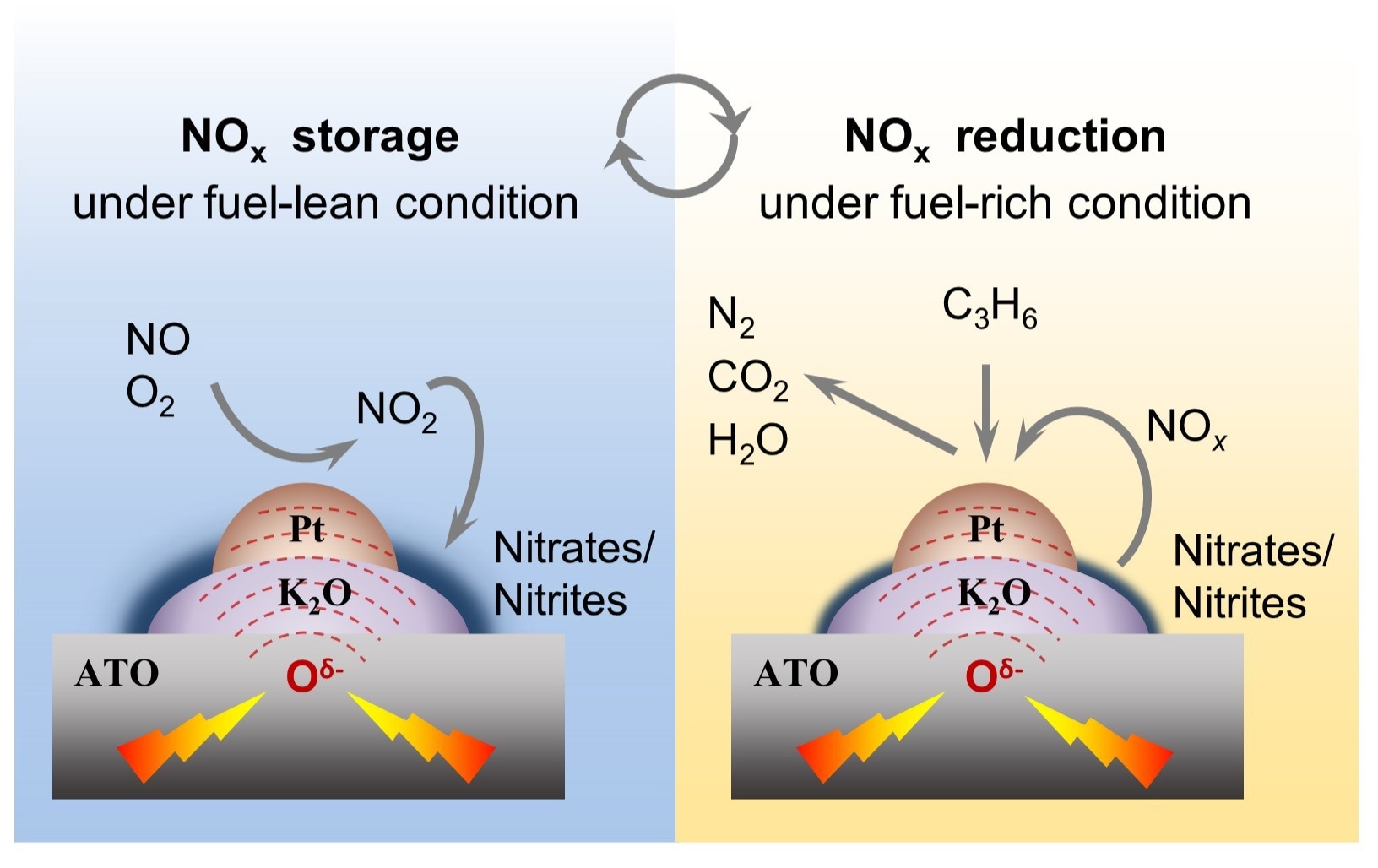
The procedure of electrified NOX storage–reduction. Image Credit: NIMTE
In order to tackle this problem, the scientists created a unique electrified NSR approach. Antimony-doped tin oxides (Pt-K/ATO) co-supported with Pt and K function as conductive catalysts. NSR reactions were triggered by applying a low input power (0.5–4 W) to the catalyst while using C3 H6 as a reductant.
This method lowered the ignition temperature for 10% NOX conversion to 165 °C, or over 100 °C below the conventional thermal counterpart.
Reducing the fuel-lean power increased the maximum energy efficiency by 23% while optimizing the power arrangement.
Furthermore, the catalysts' electrically induced discharge of lattice oxygen is demonstrated to hold pivotal functions in NSR reactions. This includes facilitating NO oxidation for NOx adsorption, promoting O2 evolution for NOx desorption, and activating C3H6 for NOx reduction. Consequently, this significantly enhances the overall NSR performance.
This effect has also been used in catalytic soot combustion, as documented in the study group's earlier work, indicating its apparent universality.
Given that the electric power supply may be changed in real-time to lower exhaust emissions, this electrification technique may provide insight into the development of electronic control units and the design of hybrid cars in general.
The study was funded by the Zhejiang Provincial Natural Science Foundation of China, the Ningbo Municipal Natural Science Foundation of China, and the Postdoctoral Science Foundation of China.
Journal Reference
Mei, X., et.al., (2023). Electrification-Enhanced Low-Temperature NOX Storage–Reduction on Pt and K Co-Supported Antimony-Doped Tin Oxides. Environmental Science & Technology. doi.org/10.1021/acs.est.3c05354.