Image Credit: Tokyo Institute of Technology
Scientists worldwide are searching for cleaner fuel substitutes for fossil fuels due to the impending threat of climate change, and many of them think hydrogen is the most suitable option. Hydrogen (H2) is a renewable energy source that does not release carbon dioxide into the atmosphere when utilized in electric power plants and automobiles.
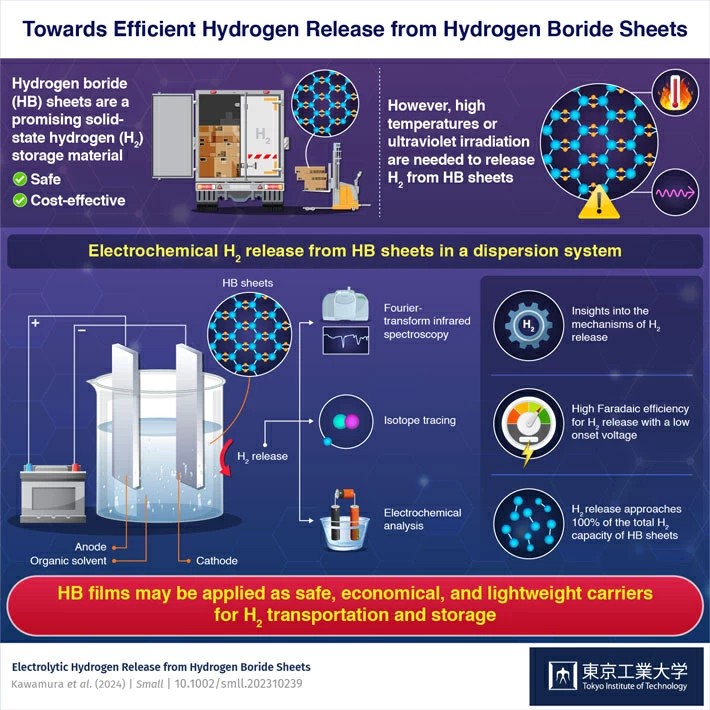
This was the focus of a team of Japanese scientists who, in a new study published in the journal Small, studied the possibility of hydrogen boride (HB) sheets as practical hydrogen carriers. Storing hydrogen in HB sheets is not a novel notion, and many elements of their possible use as hydrogen carriers have already been investigated.
However, getting the hydrogen out of the sheets is the difficult part. Heating at high temperatures or intense ultraviolet (UV) irradiation is necessary to liberate hydrogen (H2) from HB sheets. However, both systems have inherent drawbacks, such as high energy consumption or insufficient H2 production.
Thus, the researchers investigated a possible alternative: electrochemical release. Based on the mechanism of UV-induced H2 release from HB sheets, the researchers hypothesized that electron injection from a cathode electrode into HB nanosheets via an electric power source would be a better approach to release H2 than UV irradiation or heating.
Based on this assumption, the researchers dispersed HB sheets in acetonitrile, an organic solvent, and applied a regulated voltage to the mixture. These studies demonstrated that virtually all of the electrons introduced into the electrochemical system were utilized to transform H+ ions from the HB sheets into H2 molecules.
Notably, the Faradaic efficiency of this process, which evaluates the amount of electrical energy transferred into chemical energy, was greater than 90%.
The researchers also performed isotope tracing studies to show that the electrochemically generated H2 came from the HB sheets and not from any chemical process. They also used scanning electron microscopy and X-Ray photoelectron spectroscopy to analyze the sheets before and after H2 release, which provided more insights into the process's underlying mechanics.
These discoveries help to advance the creation of safe, lightweight hydrogen carriers with minimal energy usage. Although the researchers investigated the dispersed form of HB sheets in the published study, the present findings apply to film or bulk-based HB sheet systems for H2 release. In addition, the researchers will look at the rechargeability of HB sheets following dehydrogenation in a future study.
With any hope, this area of study will open the way for greener energy sources and more sustainable communities.
Journal Reference:
Kawamura, S., et. al. (2024) Electrolytic Hydrogen Release from Hydrogen Boride Sheets. Small. doi:10.1002/smll.202310239.