A research team has revealed a breakthrough in enhancing the performance of zinc-air batteries (ZABs), a significant energy storage technology, in a study published in Energy & Environmental Science.
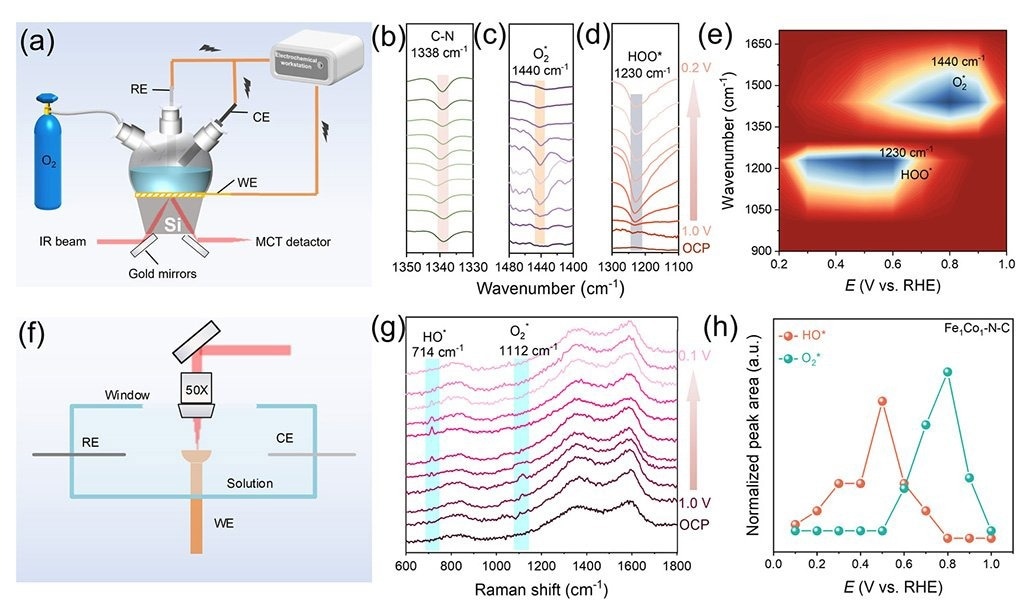 In-situ ATR-SEIRAS and in-situ Raman studies for ORR mechanism. Image Credit: Di Zhang et al.
In-situ ATR-SEIRAS and in-situ Raman studies for ORR mechanism. Image Credit: Di Zhang et al.
This discovery uses a novel catalyst that significantly enhances the oxygen reduction reaction (ORR) efficiency, a critical process in ZABs. The development could result in more efficient, long-lasting batteries for practical applications.
The oxygen reduction reaction is an important phase in many energy-conversion devices, including ZABs. However, the reaction frequently exhibits slow kinetics, limiting the performance of the batteries. Platinum-based catalysts are commonly employed to address this issue, but they are expensive, scarce, and can be contaminated with impurities.
Researchers have been looking for solutions that are both affordable and efficient. This study focuses on a novel type of catalyst known as dual-atom catalysts (DACs), which are made up of two metal atoms that are closely linked to increase catalytic activity.
Under the direction of Di Zhang, assistant professor at Tohoku University's Advanced Institute of Materials Research (WPI-AIMR), the team designed and produced a dual-atom catalyst using a combination of experimental and computational modeling methods. The catalyst is composed of iron (Fe) and cobalt (Co), which are combined with carbon (C) and nitrogen (N) in a porous structure.
It was determined that the Fe1Co1-N-C catalyst was the best one for the oxygen reduction reaction in alkaline conditions. The catalyst’s efficient operation is made possible by the special mix of materials, which makes it a viable option for ZABs.
Prior to designing the Fe1Co1-N-C catalyst, the researchers used a model to forecast the impact of pH (acidity) on the process. This helped them design a catalyst with the ideal characteristics for optimal performance.
They then created a structure with tiny pores by synthesizing the catalyst using a technique that included hard templates and a CO2 activation process. Reactants must be able to pass through these pores for the material to improve overall catalytic performance.
The findings of the investigation were impressive. The Fe1Co1-N-C catalyst demonstrated considerably better oxygen reduction activity than the frequently used platinum catalyst (Pt/C).
In practical terms, the Fe1Co1-N-C-based zinc-air batteries had a high open-circuit voltage of 1.51 volts, indicating that they can generate a significant quantity of energy. Furthermore, the batteries have an energy density of 1079 watt-hours per kilogram of zinc (Wh kgZn–1), a good indicator of energy storage capacity.
In addition to the high voltage and energy density, the Fe1Co1-N-C-based batteries displayed good rate capability, indicating that they can work well even at high current densities ranging from 2 to 600 milliamps per square centimeter (mA cm–2). More astonishingly, the batteries had a very long lifespan, lasting over 3600 hours and completing 7200 cycles at a low current, considerably outperforming most other batteries.
This work provides an efficient and rational strategy for designing and synthesizing catalysts that can be used in real-world applications. By combining theoretical models with practical synthesis methods, we were able to develop a catalyst that can significantly improve the performance of zinc-air batteries.
Di Zhang, Assistant Professor, Advanced Institute of Materials Research, Tohoku University
Zhang and his team intend to further their study by creating even more advanced methods for producing dual-atom catalysts with precise atomic pairings. They also plan to improve strategies for identifying specific active locations in catalysts. These efforts attempt to improve the performance of energy conversion technologies, making them more efficient and cost-effective for widespread application.
The Tohoku University Support Program funded the research, and significant findings are available on the Digital Catalysis Platform, a resource built by the Hao Li Lab to help in the discovery and development of novel catalysts.
Journal Reference:
Li, T., et al. (2025) A pH-dependent microkinetic modeling guided synthesis of porous dual-atom catalysts for efficient oxygen reduction in Zn–air batteries. Energy & Environmental Science. doi.org/10.1039/d5ee00215j.