DOI :
10.2240/azojomo0292
Aug 19 2009
R. Padmavathy and K. V. Rajendran
Copyright AD-TECH; licensee AZoM.com Pty Ltd.
This is an AZo Open Access Rewards System (AZo-OARS) article distributed under the terms of the AZo-OARS https://www.azom.com/oars.asp which permits unrestricted use provided the original work is properly cited but is limited to non-commercial distribution and reproduction.
AZojomo (ISSN 1833-122X) Volume 6 August 2009
Topics Covered
Abstract
Keywords
Introduction
Experimental Procedure
Result and Discussion
Conclusion
References
Contact Details
Abstract
SnO2 nanocrystaline powders with an average diameter of 12-60 nm have been successfully synthesized using different surfactants and a surface modifier, cetyltrimethylammoniumbromide (CTAB), Polyethylene glycol (PEG) and citric acid respectively at different pH via hydrothermal method. The SnO2 nanopowders were characterized using X-Ray diffractometer (XRD), UV-absorption spectroscopy, Scanning electron micrograph (SEM). XRD pattern reveals that a high crystalline rutile SnO2 nanoparticles have been synthesized. Significant morphological changes were observed in SEM due to different surfactant, surface modifier and pH. The absorption edges exhibited a blue shift, which can be ascribed to the quantum confinement effect. The probable growth mechanisms of SnO2 are discussed.
Keywords
SnO2, nanparticles, nanorods, nanopowders,
Introduction
Semiconductor nanoparticles have been extensively studied from both experimental and theoretical viewpoints, owing to their potential application in solar energy conservation, Photocatalysis and in the field of optoelectronics [1-4]. Metal oxide nanoparticles play an important role in the selective surface modification of various substrates in the form of coating. Tin oxide is a direct band gap n type semiconductor (Eg=3.6eV) and has been the most strategic material used in applications, gas sensing, transparent electrodes and liquid crystal displays etc. [5,6]. Low dimensional nanomaterials such as nanorods and nanowires are expected to have properties notably different from those of bulk material [7]. Recent studies have shown that many fundamental physical or chemical properties of semiconductor materials strongly depend on the size and morphology of the materials. Several physical and chemical synthetic methods are available for the fabrication of this material including solgel [8] CVD [9], annealing precursor powder [10], thermal evaporation and microwave heating [11]. Generally these preparation mentioned above usually involve high temperature, complex procedures, sophisticated equipment or rigorous experimental conditions Pang et.al [12] calcinated SnO2 nanocrystals at 600°C Li.et.al [9] have prepared SnO2 box beam on quartz substrate via a simple vapour deposition process at 1150°C.Mild method focus on micelle technique, electrical deposition and hydrothermal method [13]. Among these a single source hydrothermal method is simple, cost-effective, nonpolluting and energy economical method in the preparation of SnO2 nanoparticles. In this study the focus is laid on the simple and efficient hydrothermal method for the preparation of SnO2 nanoparticles and nanorods and the influence of pH, surfactants and surface modifying agent on the size, morphology and optical properties are discussed.
Experimental Procedure
SnO2 nanopowders were successfully prepared by means of dissolving 0.002 mol of SnCl2. 2H2O (A.R) in 50ml of water containing appropriate amount of NaOH. The above was stirred vigorously till a clear solution was obtained, then 0.002 mol of a CTAB was added to the above solution. After stirring the reactants were put into Teflon-lined stainless steel autoclave of 100ml capacity. The sealed autoclave was then maintained at 130°C for 24 h, and then cooled to room temperature naturally. A yellow precipitate was then collected and was washed with deionized water and absolute alcohol several times and then dried at 60°C for 3h.The obtained samples were then calcinated at 400°C for 2h. The same procedure was followed for the preparation of SnO2 nanoparticles using PEG and citric acid.
The prepared samples were subjected to different characterization including X-Ray diffraction (XRD), UV-Vis absorption spectroscopy (UV) and scanning electron micrograph (SEM). The crystalline structure of materials was analyzed by X-ray diffraction (XPERT PRO with CuKα radiation λ=1.5406Å) at scanning speed of 2°/min from 20° to 80°. The surface morphology was analyzed using Scanning electron micrograph (JEOL, JSM-67001). The absorption spectra were carried out in the range of 200 -2000nm by using SHIMDZU UV 310PC.
Result and Discussion
Fig.1 shows the XRD patterns of as synthesized SnO2 at 400°C, exhibits varied intense peaks that are easily distinguishable. The peaks were indexed as 110, 101, 200, 211, 220, 002, 310, 112, 301, 202, 321 and are in agreement with the reported value of SnO2 (JCPDS 41-1445). No characteristic peaks of impurities, such as tin metal and surfactants were observed implying the formation of pure and single-phase tin oxide. From the figure it can be noted that the peak intensity of the samples prepared using CTAB was notably higher than samples prepared by citric acid and PEG.
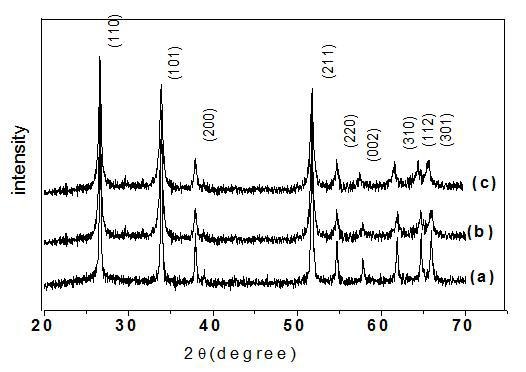
Figure 1. XRD pattern of prepared SnO2 pattern using (a) citric acid, (b) CTAB, (c) PEG at pH=2.
The crystalline sizes obtained using different surfactants, surface modifier at different PH were calculated using Debye Scherer's equation [14] and listed in Table 1.
Table1. Grain Size and Morphology of SnO2 nanoparticles synthesized using surface modifier, cationic and non-ionic surfactant at different pH
|
Category
|
|
|
|
|
Surface modifier
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cationic surfactant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-ionic surfactant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 2 represent the single XRD peak of the sample using citric acid to obtain the value of full wave half maxima â which was found to be 1.26
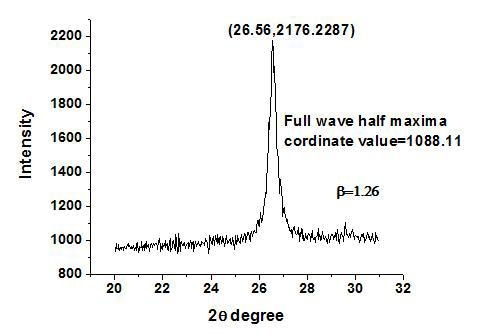
Figure 2. Single XRD peak of prepared SnO2 pattern using citric acid.
The absorption spectra of the samples obtained from different surfactant and surface modifying agent were shown in Fig. 3 exhibits a blue shift in the absorption band edge which could be attributed to well known quantum size effect of semiconductor.
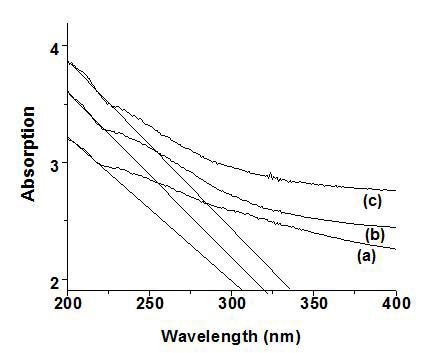
Figure 3. Absorption spectra of synthesized SnO2 using (a) citric acid, (b) CTAB, (c) PEG at pH=2.
One of the key factors associated with the nanomaterial is the size dependence of their physical and chemical properties. As the small size of nanoparticles, result in spatial confinement of the charge carrier wave function, which is termed as quantum size effect. The quantum size effects not only include blue shift of the absorption edge and exciton energy but it also cover the increase in exciton association strength and biding energy [15]. Considering the blue shift of the absorption position from the bulk SnO2, the absorption onsets of the present samples can be assigned to the direct transition of electron in SnO2 nanocrystals [16]. In order to obtain the band gap value the slope was drawn to the higher wavelength region as reported. The sample synthesized using surface modifier exhibited a absorption edge at 310 nm which is blue shifted considerably compared with the samples prepared using cationic (316 nm) and non-ionic surfactants (332 nm). From the graph (Fig. 4) it can be observed that the grain size and band gap varies linearly, implying that as the grain size increases the absorption edge is shifted towards the higher wavelength region. As pH varies from 2 to 7 the absorption spectra is shifted towards red implying larger particle size at higher value of pH.
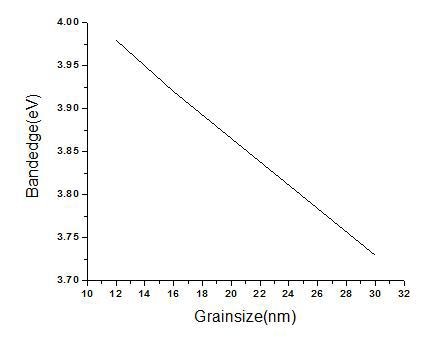
Figure 4. Relation between grain size and band gap.
Fig. 5 depicts the SEM images of SnO2 nanoparticles synthesized by using two different surfactants, and a surface modifier (CTAB, PEG, Citric acid) at three different pH, which have a great influence in determining the morphology and size of SnO2 nanoparticles. In the case of surface modifying agent citric acid no distinct morphological changes (spherical shape) were observed but exhibited variations in the grain size at different pH values. In the case of cationic surfactant CTAB, the electrostatic interaction takes place between CTA+ cations and Sn(OH)62- the cation CTA+ condense into aggregates in which counterions Sn(OH)62- anions are interrelated in the interfaces between the head group to form CTA+- Sn(OH)62- pair[14]. Morphological changes were observed by increasing the value of pH. Spherical shape nanoparticles were observed at pH=2 whereas both particles and rods at pH=7 and the formation of rod could be due to the oriented growth aggregation of nanoparticles. PEG being a non-ionic surfactant SnO2 formation is not possible by electrostatic interaction, and the formation can be attributed to weak Vanderwall's interaction[17]. Spherical shape was observed at pH=2 and then modified into cauliflower like shape at pH=5 latter exhibited a triangular shape for pH=7. The change in the morphology with different surfactants, surface modifying agent and pH are listed in table1.
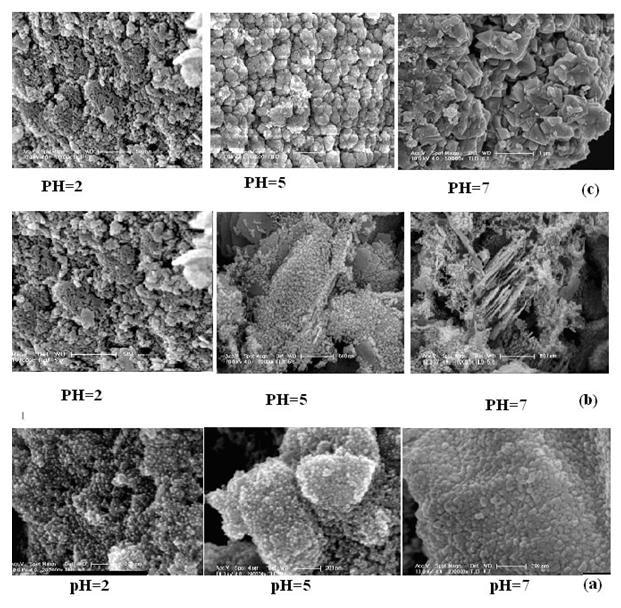
Figure 5. SEM images of SnO2 using (a) citric acid, (b) CTAB, (c) PEG at different pH values.
Conclusion
SnO2 nanoparticles having particle sizes from 12 - 60 nm were productively synthesized by a simple hydrothermal method. XRD results showed that the nanoparticles and rods were single crystalline SnO2 with rutile structure. SEM photographs exhibits different morphologies for different pH and surfactants. The absorption edge showed a prominent blue shift. From the above discussion it can be concluded that SnO2 nanoparticles prepared using citric acid a surface modifier showed a lesser particle size compared to the samples prepared from CTAB a cationic surfactant and PEG a nonionic surfactant. This convenient synthesis strategy can be applied as general approach for the preparation of other metal oxides nanoparticles and nanorods.
References
- Raghumani Singh Ningthoujam and S. K. Kulshreshtha, "Nanocrystalline SnO2 from thermal decomposition of tin citrate crystal: Luminescence and Raman studies"., Materials Research Bulletin, 44 (2009) 57-62.
- G. Ansari, D. Boroojerdian, S. R. Sainker, R. N. Karekar, R. C. Aiyer and S. K. Kulkarni, "Grain size effects on H2 gas sensitivity of thick film resistor using SnO2 nanoparticles"., Thin solid films, 295 (1997) 271-276.
- X. Peng, M. C. Schlamp, A. V. Kadavanich, and A. P. Alivisatos, "Epitaxial growth of highly luminescent cdSe/CdS core/shell nanocrystals with photostability and electron accessibility"., J. Am. Chem. Soc., 119 (1997) 7019-7029.
- Wan - Young Chung, Duk - Dong Lee and Byung - Ki Sohn, "Effects of added TiO2 on the characteristics of SnO2 - based thick film gas sensors"., Thin solid films, 221 (1992) 304-310.
- H. Ogawa, A. Abe, M. Nishikawa and S. Hayakawa, "Electrical properties of tin oxide ultrafine particle films"., J. Electrochem. Soc., 128 (1981) 2020-2025.
- Z. M. Jarzebski and J. P. Marton, "Physical properties of SnO2 materials"., J. Electrochem. Soc., 123 (1976) 299C-310C.
- Jiesen Wang, Jionquan Sun, Guosong Zhang, Xiuchun Wu, Ying bao and Hui Li Dairong Chen, "Preparation of SnO2 nanorods via oriented aggregation of nanoparticles"., Vacuum 82, (2008) 5-8.
- L. B. Fraigi, D. G. Lamas and N. E. Walsoede Reea, "Comparison between two combustion routes for the synthesis of nanocrystalline SnO2 powders"., Mater. Lett., 47 (2001) 262-266.
- Y. Liu, J. Dong and M. Liu, "Well - Aligned Nano - Box - Beams of SnO2"., Adv. Mater., 16 (2004) 353-356.
- Yingkai Liu, Weiguo Yang, Zhifu Dai, Haiyan Chen, Xiaoli Yang and Dedong Hou, "s Improved Molten state synthesis of SnO2 nanorods and nanotwins".,Materials Chemistry and Physics, 112 (2008) 381-386.
- J. J. Zhu, J. M. Zhu, X. H. Liao, J. L. Fang, M. G. Zhou and H. Y. Chen, "Rapid synthesis of nanocrystaline SnO2 powders by microwave heating method"., Mater. Lett., 53 (2002) 12-19.
- G. Pang, S. Chen, Y. Koltypin, A. Zaban, S. Feng and A. Gedanken, "Controlling the particle size of calcinated SnO2 nanocrystals"., Nano Lett., 1, (2001) 723-726.
- D. F. Zhang, L. D. Sun, J. L. Yin and C. H. Yan, "Low-Temperature Fabrication of Highly Crystalline SnO2 Nanorods"., Adv. Mater., 15 (2003) 1022-1025.
- Azam Anaraki Firooz, Ali Reza Mahjoub and Abbas Ali Khodadadi, "Preparation of SnO2 nanoparticles and nanorods by using hydrothermal method at low temperature"., Materials Lett., 62 (2008) 1789-1792.
- He Hu and Weihua Zhang, "Synthesis and properties of transition metals and rare earth metals doped ZnO nanoparticles"., Optical materials, 28 (2006) 536-560.
- Feng Gu, Shu Fen Wang, Meng Kai Lu, Yong Xin Qi, Guangjun Zhou, Dong Xu and Duio Rong Yuan, "Luminescent properties of Mn2+- doped SnO2 nanoparticle"., Inor. Chem. Commu., 6 (2003) 82-885.
- Li Yan, Yadong Li, Zhao - Xiang Deng, Jinzhuang, Xiaoming Sun, "Surfactant - assisted hydrothermal synthesis of hydroxypatite nanorods"., Inter. J. Inorganic Materials, 3 (2001) 633-637.
Contact Details
R. Padmavathy and K. V. Rajendran
Presidency College,
Chennai-600 005,
Tamil Nadu,
India
E-mail: [email protected]
This paper was also published in print form in "Advances in Technology of Materials and Materials Processing", 11[1] (2009) 31-36.