Jun 8 2016
Since the theoretical capacity of calcium ion batteries (CIBs) is double that of lithium ion batteries (LIBs), CIBs have garnered more attention as futuristic batteries to substitute LIBs. This increase in capacity is due to the difference between divalent and monovalent ions. Additionally, CIBs are cheaper and safer, as calcium is present in more quantities than lithium and the melting point of CIBs is also higher than that of LIBs.
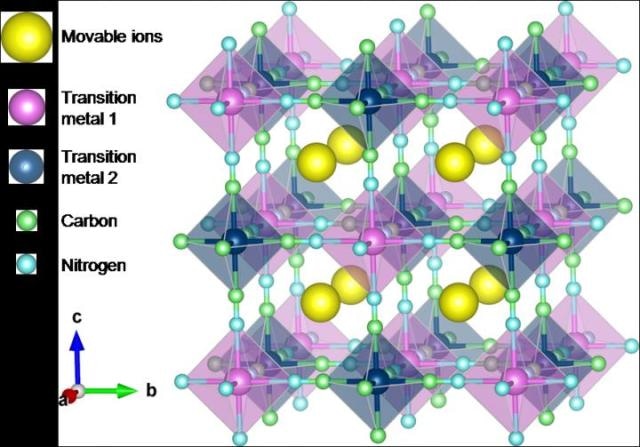 Crystal structure of Prussian blue and its analogues. (Credit- Toyohashi University of Technology)
Crystal structure of Prussian blue and its analogues. (Credit- Toyohashi University of Technology)
Nevertheless, a major drawback in CIBs is that there is no appropriate electrode material in which addition and removal of calcium ions can be carried reversibly due to the larger ionic radius of calcium ions (112 pm) when compared to lithium ions (76 pm).
Tomohiro Tojo and his co-workers at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, used Prussian blue (PB) and Prussian blue analogues (PBAs) as CIB electrodes since they contain large sites for introducing and removing large-sized ions. Till date, the electrochemical behaviors of sodium ions equivalent to the radius of calcium ions have been analyzed in inorganic and organic electrolytes using PBAs as electrode material. These studies have shown reversible extraction and insertion of sodium ions to and from PBA structures.
The researchers explored the electrochemical performance of a number of PBA electrodes to find if calcium ions in an organic electrolyte show irreversible or reversible extraction and insertion into and from the crystal structure. It was found that there were reversible capacities of 40 to 50 mAh/g at less current density (Figure 2(a). In Figure 2(b), the Coulombic efficiencies, defined as the ratio of the amount of insertion (discharge) and extraction (charge) of calcium ions, were found to exhibit a constant value of 90% following the 3rd cycle.
The results shown in Figure 2 show the exceptional cyclability of the PBA electrodes, although the theoretical capacity was double the reversible capacities. The scientists explored the reason behind the high reversibility by utilizing X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). This reversibility can be elucidated by the strong structure of the PBAs and their exceptional charge balance while inserting and extracting the calcium ions.
Prussian blue (PB) and Prussian blue analogues (PBAs) were proposed to be viable electrode materials for CIBs, although further investigation is required to further improve the reversible capacities. Further study has been carried out by the scientists to use CIBs beyond LIBs.