Jul 20 2016
It is not possible for an electron microscope to take a photo like a mobile phone camera. An electron microscope’s ability to image a structure, and the success of the imaging will depend on the researcher’s understanding of the structure. In most cases, complex physics calculations are required to make complete use of the electron microscopy’s potential.
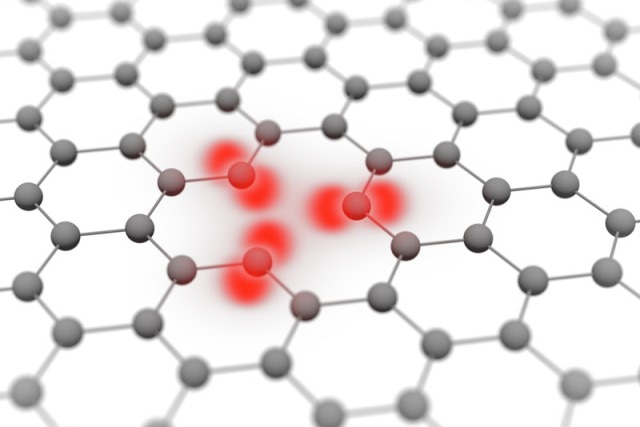 Atomic orbitals of carbon atoms in graphene (Photo credit: TU Wein)
Atomic orbitals of carbon atoms in graphene (Photo credit: TU Wein)
TU Wien’s Prof. Peter Schattschneider headed an international research team that was involved in analyzing the opportunities offered by energy-filtered transmission electron microscopy (EFTEM).
With the right conditions, the team could illustrate numerically that it was feasible to procure clear images of the orbital of every single electron within an atom. The electron microscopy can be used to penetrate down to the subatomic level. Many experiments are already being planned in this area.
The research has been published in the physics journal “Physical Review Letters”.
In search of the electron orbital
People often picture atomic electrons as tiny spheres circling around the nucleus of the atom similar to miniature planets around a sun. However, this image is hardly reflected in reality. The laws of quantum physics prescribe that an electron’s position cannot be unmistakably defined at any given point in time. The electron is efficiently smeared across an area near to the nucleus.
The area where the electron could probably be found is called the orbital. So far only the shape of these orbitals could be calculated. However, attempts to image them with electron microscopes have not been successful until now.
We have calculated how we might have a chance of visualizing orbitals with an electron microscope. Graphene, which is made of just one single layer of carbon atoms, is an excellent candidate for this task. The electron ray is able to pass easily through the graphene with hardly any elastic scattering. An image of the graphene structure can be created with these electrons.
Stefan Löffler, University Service Centre for Transmission Electron Microscopy (USTEM), TU Wien
The principle of EFTEM has been known to researchers for some time. EFTEM can be used to develop fairly specific visualizations of specific kinds of atoms even as others are blocked. Therefore, it is frequently used to study the chemical composition of microscopic samples.
“The electrons shot through the sample can excite the sample’s atoms”, explains Stefan Löffler. “This costs energy, so when the electrons emerging emerge from the sample, they are slower than when they entered it. This velocity and energy change is characteristic for certain excitations of electron orbitals within the sample."
Once the electrons pass through the sample, a magnetic field arranges the electrons by energy.
"A filter is used to block out electrons that aren’t of interest: the recorded image contains only those electrons that carry the desired information.”
Defects can be helpful
The researchers used simulations to examine how this method could help realize a turning point in the research of electron orbitals. While working on this, they stumbled upon something that in fact facilitated the imaging of each orbital:
The symmetry of the graphene has to be broken. If, for instance, there is a hole in the graphene structure, the atoms right beside this hole have a slightly different electronic structure, making it possible to image the orbitals of these atoms. The same thing can happen if a nitrogen atom rather than a carbon atom is found somewhere in the graphene. When doing this, it’s important to focus on the electrons found within a narrow and precise energy window, minimise certain aberrations of the electromagnetic lens and, last but not least, use a first-rate electron microscope.
Stefan Löffler, University Service Centre for Transmission Electron Microscopy (USTEM), TU Wien
All of these problems can be resolved, however, as the team’s calculations illustrate.
The Humboldt-Universität zu Berlin, the Universität Ulm, and McMaster University in Canada took part in the TU Wien study - FWF-DFG project (“Towards orbital mapping”, I543-N20) and a FWF Erwin-Schrödinger project (“EELS at interfaces”, J3732-N27).
At present, Ulm is working on a new, high-performance transmission electron microscope that will be used to transform these ideas into reality in the near future. Preliminary results have surpassed expectations.