Dec 2 2016
 (Credit: NC State University)
(Credit: NC State University)
A team of researchers from North Carolina State University, Duke University and Brookhaven National Laboratory have discovered that molybdenum sulfide (MoS2) has more potential than formerly thought as a catalyst for creating hydrogen to use as a clean energy source. The researchers specifically found that the whole surface of MoS2 can be used as a catalyst, not merely the edges of the material.
The key finding here is that the intrinsic catalytic performance of MoS2 is much better than the research community thought. We’re optimistic that this can be a step toward making hydrogen a larger part of our energy portfolio.
Linyou Cao, Associate Professor, NC State
Hydrogen promises clean energy, producing just water as a byproduct. But to produce hydrogen for use as a clean energy source, ideally the hydrogen gas would be able to be isolated from water – with oxygen being the only byproduct.
However, the solution to producing hydrogen from water – a process known as hydrogen evolution – is an efficient catalyst. Currently, platinum is the best catalyst, but it is too expensive for large scale use.
Another option for a hydrogen evolution catalyst is MoS2, which is both abundant and inexpensive. However, it has long been thought that MoS2 has limited use, based on the conventional understanding that only the edges of MoS2 serve as catalysts and not the bulk of the material, which is left inactive.
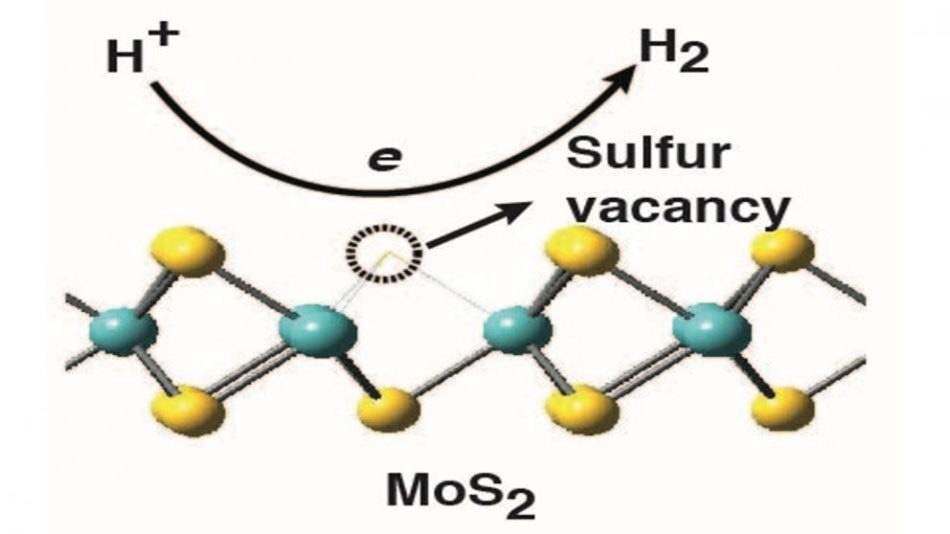
The new research from NC State, Duke and Brookhaven demonstrates that the surface of MoS2 can be engineered to capitalize on the material’s catalytic efficiency. And the key to this efficiency is the number of sulfur vacancies present in the MoS2.
Imagine the crystalline structure of MoS2 to be a grid of regularly spaced sulfur and molybdenum atoms. When one of those sulfur atoms is missing, a sulfur vacancy occurs.
We found that these sulfur vacancies attract the hydrogen atoms in water at just the right strength: the attraction is strong enough pull the hydrogen out of the water molecule, but is then weak enough to let the hydrogen go.
Linyou Cao, Associate Professor, NC State
The team also discovered that the grain boundaries of MoS2, which have been thought to be catalytically active for hydrogen evolution by the science community, may only provide slight activity. The boundaries between crystalline domains are referred to as grain boundaries.
These findings take researchers in a new direction for optimizing the catalytic performance of MoS2. Currently, the most common approach is to increase the number of edge sites, as the conventional knowledge dictates that only the edge sites are catalytically active.
Our result indicates that grain boundaries should not be the factor to consider when thinking about improving catalytic activity. The best way to improve the catalytic activities is to engineer sulfur vacancies. The edges of MoS2 are still twice as efficient at removing hydrogen atoms compared to the sulfur vacancies. But it’s difficult to create a high density of edges in MoS2 – a lot of the material’s area is wasted – whereas a large number of sulfur vacancies can be engineered uniformly across the material.
Linyou Cao, Associate Professor, NC State
Additionally, the researchers found that there is a “sweet spot” for increasing the catalytic efficiency of MoS2.
“We get the best results when between 7 and 10 percent of the sulfur sites in MoS2 are vacant,” Cao says. “If you go higher or lower than that range, catalytic efficiency drops off significantly.”
The team noticed that the crystalline quality of MoS2 is key to optimize the catalytic activity of the sulfur vacancies. The sulfur vacancies in high crystalline quality MoS2 displayed better efficiency compared to those in low crystalline quality MoS2, even in cases where the densities of the vacancies were the same.
“In order to get the best output from sulfur vacancies, the crystalline quality of MoS2 needs to be very high,” says Guoqing Li, a Ph.D. student at NC State and lead author of the paper. “The ideal scenario would be 7 to 10 percent sulfur vacancies uniformly distributed in a single crystalline MoS2 film.”
The research was performed using MoS2 thin films that were just three atoms thick. With these engineered thin films, the team was able to realize catalytic efficiency similar to earlier MoS2 technologies that depended upon having two or three orders of magnitude more surface area.
We now know that MoS2 is a more promising catalyst than we anticipated, and are fine-tuning additional techniques to further improve its efficiency. Hopefully, this moves us closer to making a low-cost catalyst that is at least as good as platinum.
Linyou Cao, Associate Professor, NC State
The paper, “All the Catalytic Active Sites of MoS2 for Hydrogen Evolution,” is published in the Journal of the American Chemical Society. The paper was co-authored by Yifei Yu, David Peterson, Abdullah Zafar, Raj Kumar, Frank Hunte and Steve Shannon of NC State; Du Zhang, Stefano Curtarolo and Weitao Yang of Duke; and Qiao Qiao and Yimei Zhu of Brookhaven National Lab.
The research was conducted with support from the Department of Energy’s Office of Science, under grants DE-SC0012575 and DE-SC0012704, as well as by the National Science Foundation under grant PHY1338917.