Mar 24 2017
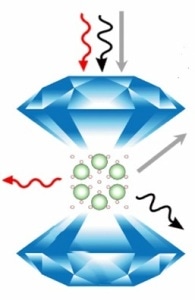 An illustration of Ar(H2)2 in the diamond anvil cell. The arrows represent different ways that spectroscopic tools study the effect of extreme pressures on the crystal structure and molecular structure of the compound. (For experts, the red arrow represents Raman spectroscopy, the black arrow represents synchrotron x-ray diffraction, and the gray arrow represents optical absorption spectroscopy.) Image courtesy of Cheng Ji.
An illustration of Ar(H2)2 in the diamond anvil cell. The arrows represent different ways that spectroscopic tools study the effect of extreme pressures on the crystal structure and molecular structure of the compound. (For experts, the red arrow represents Raman spectroscopy, the black arrow represents synchrotron x-ray diffraction, and the gray arrow represents optical absorption spectroscopy.) Image courtesy of Cheng Ji.
Studying hydrogen can teach scientists about the fundamental nature of matter as hydrogen is both the most-abundant and the simplest element in the universe. However, there are still a number of hydrogen secrets that are yet to be unlocked, including the best way that can be used to force hydrogen into a superconductive, metallic state with no electrical resistance.
Although theoretically ideal for energy transfer or storage, metallic hydrogen is extremely challenging to produce experimentally.
Ho-kwang “Dave” Mao
It has been earlier proposed that the introduction of impurities into a sample of molecular hydrogen, H2, could help to simplify the transition to a metallic state. Therefore, Mao and his team focused on studying the intermolecular interactions of hydrogen that are weakly-bound, or “doped,” with argon, Ar(H2)2, under intense pressures.
The idea highlighted here is that the nature of the bonds between the hydrogen molecules could be changed by the impurity, decreasing the pressure needed to induce the nonmetal-to-metal transition. The fact that Ar(H2)2 could be a good candidate was indicated by earlier research.
To their surprise, the researchers observed that the addition of argon failed to facilitate the molecular changes that are essential for initiating a metallic state in hydrogen. Their findings are featured in the Proceedings of the National Academy of Sciences.
The researchers brought the argon-doped hydrogen up to 3.5 million times normal atmospheric pressure (or 358 gigapascals) inside a diamond anvil cell and studied its structural changes with the help of enhanced spectroscopic tools.
The team discovered that hydrogen remained in its molecular form even in the most highest pressures, establishing the fact that argon is not actually the facilitator that many believed it would be.
Counter to predictions, the addition of argon did not create a kind of ‘chemical pressure’ on the hydrogen, pushing its molecules closer together. Rather, it had the opposite effect.
Cheng Ji, Lead Author
The co-authors of the study include Carnegie’s Alexander Goncharov, Dmitry Popov, Bing Li, Junyue Wang, Yue Meng, Jesse Smith, and Wenge Yang; as well as Vivekanand Shukla, Naresh Jena, and Rajeev Ahuja of Uppsala University; and Vitali Prakpenka of the University of Chicago.
This research received support from the U.S. National Science Foundation, the U.S. Department of Energy, the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists and Recruitment of Foreign Experts, the European Erasmus Fellowship program, and the Swedish Research Council.