Apr 11 2017
 A slight change in the structure of the organocatalyst generates the other diastereomer in high efficiency. Credit: ITbM, Nagoya University
A slight change in the structure of the organocatalyst generates the other diastereomer in high efficiency. Credit: ITbM, Nagoya University
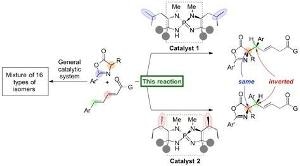
Nagoya University researchers have presented a report in Nature Communications highlighting the development of an organic catalyst (organocatalyst) that activates a highly stereoselective 1,6-addition of azlactones (nucleophile) to a δ-aryl dienyl carbonyl compound (electrophile) to produce high yields of amino acid derivatives. The generated 1,6-adduct comprises of two carbon stereocenters, and a slight structural change in the organocatalyst results in inversion of stereochemistry at a single stereocenter to develop a diastereomer in high selectivity. This research commenced in 2012 and the researchers serendipitously discovered this inversion of stereochemistry upon screening a wide range of amino acids, which are used in their unique iminophosphorane catalyst.
Stereocenters are chiral centers, where an atom comprises of three or more different atoms or functional groups fixed to it. A number of molecules with pharmaceutical uses comprise of these stereocenters and the development of effective stereoselective reactions in order to synthesize a specific stereoisomer (isomers that vary in the three-dimensional orientations of their atoms in space) is in great demand. The reason for this is that each stereoisomer generally has a variety of characteristics and precise control is needed in order to attain the desired stereoisomer in a pure form.
A series of stereoisomers is obtained when connecting carbon atoms that have three different functional groups attached to them, and these functional groups are orientated in a diffident manner in space.
Enantiomers are a specific type of stereoisomer, in which they comprise of one or more stereocenters and are mirror images of each other. Till date, a number of asymmetric reactions have been created to develop chiral centers in high efficiency. On the other hand, diastereomers are referred to as stereoisomers of a compound with varied configurations at one or more stereocenters and are not mirror images of each other.
Connecting a pair of carbon molecules, each having two different hands, allows these molecules to be connected in a wide range of combinations, and it is possible to synthesize four different stereoisomers in theory. These stereoisomers are a series of diastereomers and enantiomers based on the relationship to each other (mirror image or not). Traditional methods to synthesize diastereomers have needed a particular catalyst for each isomer. A totally new catalytic system is needed in most cases in order to specifically attain one of the stereoisomers.
When each of the two molecules to be connected is available with four different hands, the situation becomes more complex and potentially leads to 16 (24) types of stereoisomers. The possible generation of regioisomers (positional isomers) also arises since the reaction can now take place at different positions. The development of a specific stereoisomer (diastereomer, enantiomer, or regioisomer) will require a reaction system to be established for the starting materials to react at a particular site and in a specific orientation, i.e. for the molecules to be located to hold hands in a specific manner.
Professor Takashi Ooi's group at the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University has come up with a new protocol in which the researchers have developed iminophosphorane catalysts capable of generating specific stereoisomers in high yield and selectivity. Additionally, a minor change in the organocatalyst structure leads to pinpoint inversion of a single stereocenter in order to produce a diastereomer, allowing access to a specific diastereomer of interest in a pure form.
I was really excited the moment I saw the inversion in stereochemistry by changing the organocatalys. Initially, we were trying to expand the scope of our catalytic system to new substrates, so this was also the moment when I thought that this was more than an ordinary stereoselective reaction.
Ken Yoshioka, Graduate Student, Nagoya University
The iminophosphorane catalyst is obtained from amino acids, and the properties of the catalyst can be tuned when a change is brought about in the amino acid structure. In this scenario, a small modification in the position of the methyl groups on the catalyst will result in the diastereomer of the 1,6-adduct.
"Since starting this research 5 years ago, it took me about 3 years to find the optimal reaction conditions after finding the stereochemical inversion reaction," continues Yoshioka. "One main issue was the reproducibility of this reaction, as the selectivities varied in each reaction. I had repeated the reaction over and over again to see what was happening."
"We were really confused by these variable results and we initially assumed that the presence of water was playing a role in the transition state and was affecting the selectivity of this reaction," says Daisuke Uraguchi, an Associate Professor at Nagoya University. Complete removal of water is not an easy task in organocatalysts as they can develop hydrogen bonds with water molecules.
After various optimization studies, we were able to find that lowering the temperature to 30 °C was the key to controlling the selectivity of this 1,6-addition reaction. This took a while to figure out, and were relieved to be able to generate reliable results. We were also able to stereospecifically synthesize diversely functionalized proline derivatives by further reactions of the 1,6-adducts.
Ken Yoshioka, Graduate Student, Nagoya University
"We then carried out experimental and computational studies to find a rationale for this unique stereochemical inversion," explains Uraguchi. "The organocatalysts that lead to different diastereomers share the same core and we were keen to find out how the position of the methyl groups on the catalyst affects the diastereoselectivity of this reaction."
Analysis by density functional theory (DFT) and X-ray crystallography studies highlight that the shape of the catalyst plays a vital role in positioning the substrates for reacting with one another. “Even though the methyl groups appear to be on the outside of the catalyst, they actually have a huge influence on holding the substrates in place to react on a particular face," describes Uraguchi. "We were able to show that a small difference in the catalyst structure changes the transition state, and leads to a change in diastereoselectivity.”
Diastereodivergence (developing diastereomers from a common set of substrates) is considered to a challenging topic, however the team succeeded in producing a new strategy for the inversion of stereochemistry by their unique reaction system. "The key to the success of this work was to keep challenging on difficult topics and to question any small observation," says Uraguchi. "Ken Yoshioka worked extremely hard on this project, and I believe that if it wasn't for him, we wouldn't have gone this far."
I had been working on this project throughout the course of my graduate studies and I believe that my persistence paid off. Although there were times where we were unsure of what was happening in the reaction, we checked each factor one by one and it was a great feeling of satisfaction to find the origin of the stereoselectivity.
Ken Yoshioka, Graduate Student, Nagoya University
"We were pleased to accomplish diastereodivergence in 1,6-addition reactions with high levels of stereocontrol, and envisage that this diastereodivergent strategy will advance the field of asymmetric synthesis," says Uraguchi and Takashi Ooi, a Professor at Nagoya University, who led this study. "We hope to continue to make unique catalysts that will contribute to making complex molecules, which will have potential uses in the pharmaceutical and agrochemical industries," says Ooi.