Jun 30 2021
At the University of Leicester, scientists have designed a new technique to recycle electric vehicle batteries with the help of an innovative approach that many people might have experienced in the dentist’s chair.
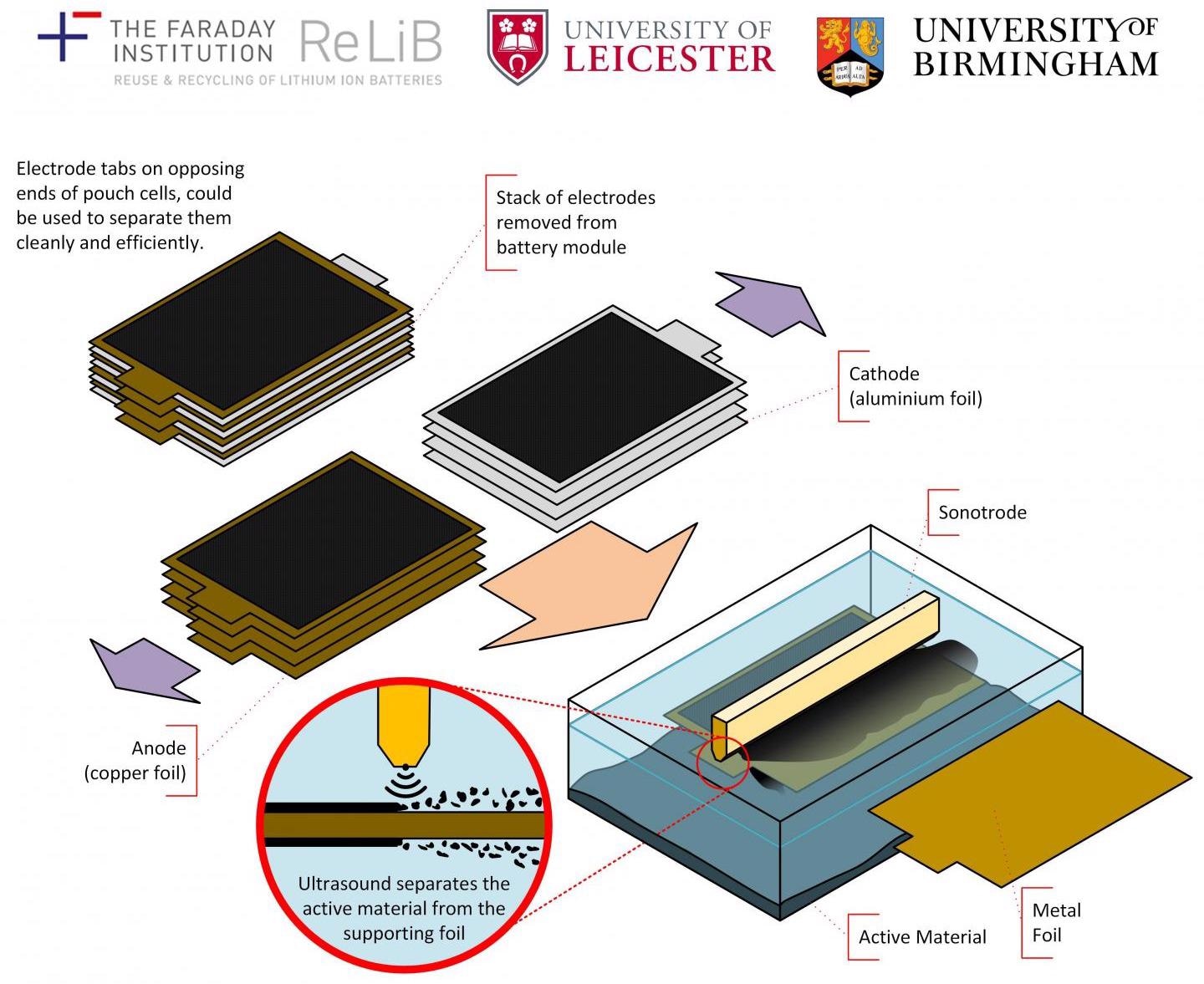 Diagram showing the ultrasonic process which assists in the delamination of lithium-ion batteries. Image Credit: University of Leicester.
Diagram showing the ultrasonic process which assists in the delamination of lithium-ion batteries. Image Credit: University of Leicester.
Headed by Professor Andy Abbott from the University of Leicester, the Faraday Institution project on the recycling of lithium-ion batteries (ReLiB) has utilized a new technique, including ultrasonic waves, to find a solution to a major challenge: how to isolate useful materials from electrodes so that the materials can be completely recovered from batteries at the end of their life.
Existing techniques for recycling lithium-ion batteries normally involve feeding end-of-life batteries into a high-temperature reactor or shredder. A complicated set of chemical and physical processes are then required to generate usable materials. Such recycling routes are inefficient and energy-intensive.
If a different method is used and end-of-life batteries are disintegrated instead of being shredded, there is the possibility of recovering more material, in a purer state. The disintegration of lithium-ion batteries has been demonstrated to recover a high yield (nearly 80% of the original material) in a purer state compared to what was possible with shredded material.
The faltering block — of how to eliminate and isolate crucial materials (like cobalt, manganese nickel, and lithium) from used-up batteries in a quick, cost-effective and eco-friendly manner — can now be circumvented by using the new approach, which adapts technology that is largely used in the current food preparation sector.
The method of ultrasonic delamination helps to effectively blast the active materials needed from the electrodes, with copper or virgin aluminum left behind. The process proved to be highly effective in eliminating lithium nickel manganese cobalt oxides — often called NMC — and graphite.
The research team, headed by Professor Abbott, has applied for a patent for the method. The study has been reported in the journal Green Chemistry.
This novel procedure is 100 times quicker and greener than conventional battery recycling techniques and leads to a higher purity of recovered materials. It essentially works in the same way as a dentist’s ultrasonic descaler, breaking down adhesive bonds between the coating layer and the substrate.
Andy Abbott, Professor, University of Leicester
“It is likely that the initial use of this technology will feed recycled materials straight back into the battery production line. This is a real step change moment in battery recycling,” added Abbott.
According to Professor Pam Thomas, CEO of the Faraday Institution, “For the full value of battery technologies to be captured for the UK, we must focus on the entire life cycle — from the mining of critical materials to battery manufacture to recycling — to create a circular economy that is both sustainable for the planet and profitable for industry.”
The focus of scientists at the Faraday Institution has been on the battery’s lifecycle — from their initial production to their re-use in secondary applications to their ultimate recycling, to guarantee that the economic and environmental benefits from Electric Vehicle batteries are completely realized.
The researchers are in their first phase of discussions with various recycling companies and battery manufacturers to place a technology demonstrator at an industrial site in 2021, with a longer-term aim of licensing the technology.
Additionally, the research group has tested the technology on the four most regular battery types and discovered that it exhibits a performance with the same efficiency in every single case.
Journal Reference:
Lei, C., et al. (2021) Lithium-ion battery recycling using high-intensity ultrasonication. Green Chemistry. doi.org/10.1039/D1GC01623G.