Lithium–sulfur batteries hold an immense amount of potential as next-generation energy storage devices due to their ultrahigh theoretical energy density.
 Scientists created an effective redox mediator to promote the sulfur redox kinetics under practical working conditions. Image Credit: Nano Research, Tsinghua University Press.
Scientists created an effective redox mediator to promote the sulfur redox kinetics under practical working conditions. Image Credit: Nano Research, Tsinghua University Press.
But the actual energy density of lithium–sulfur batteries seems to be distant from the theoretical value due to the severe limitations of the cathode as a result of the slow sulfur oxidation–reduction (redox) reactions’ kinetics.
The research group has made a redox mediation strategy that is efficient and drives lithium–sulfur batteries toward practical application.
The research group reported their findings in the Nano Research journal on June 21st, 2022.
At present, lithium batteries are extensively utilized in applications varying from portable electronics, and electric vehicles to grid-scale energy storage systems. Amongst the several lithium batteries, lithium-ion batteries are the most popular.
But lithium-ion batteries are approaching their theoretical energy density limit and cannot emulate the needs of high-energy-density energy storage devices.
The lithium–sulfur batteries, along with their ultra-high theoretical energy density of 2600 Wh kg−1, are gaining huge attraction as a possible alternative to the lithium-ion batteries
In addition to their high-energy-density potential, the sulfur utilized as the active material in the cathode seems to be affordable, eco-friendly, and naturally abundant.
But there is a considerable hindrance to overcome. The high theoretical energy density in lithium–sulfur batteries occurs from the reactions between the lithium anode and the sulfur cathode.
At the time of the discharge process, when the conversion of chemical energy to electrical energy has been done, the sulfur is dissolved into lithium polysulfides and further to solid lithium sulfide.
However, when this conversion process happens, the kinetics, or rate of the chemical reactions, is quite slow. This sluggishness would be further provoked under rough practical working conditions.
To resolve the challenge of sluggish kinetics, scientists have developed a range of promoters to enhance the reactions of the battery. Such promoters tend to act as sulfur hosts or interlayer materials in the lithium–sulfur batteries and are designed to encourage the kinetics of the batteries.
But even with the help of such promoters, the performance of the battery drops slowly due to the solid lithium sulfide deposits that tend to accumulate on the electrocatalytic active sites.
With additional studies, scientists have identified that soluble redox mediators are efficient in encouraging kinetics. Redox, short for oxidation–reduction, explains the chemical reactions that occur. Such redox mediators chemically decrease or oxidize the lithium polysulfides and further regenerate at the electrode’s surface.
Scientists have proven that making use of the redox mediators is definitely an effective mechanism for tackling the sluggish kinetics present in coin cell batteries. Furthermore, coin cells, also known as button cells, are compact, flat batteries that find their applications in smaller devices like car keys, hearing aids, or medical implants.
What was required next was a redox mediator that functioned in pouch cell lithium batteries, which are utilized in higher power applications, like automotive and military applications.
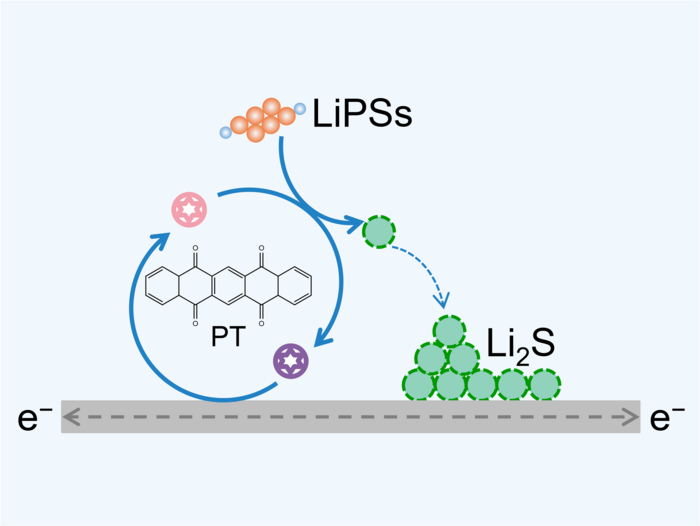
The research group saw the need to develop an advanced redox mediator that is ideal for practical working lithium–sulfur pouch cells as urgent. Hence, the research team developed a redox mediator with the help of an organic molecule known as 5,7,12,14-pentacenetetrone or PT. This helps to encourage the sulfur redox kinetics in high-energy-density lithium–sulfur pouch cells.
Concretely, the PT redox mediator provides a chemical bypass for the reduction of lithium polysulfide to lithium sulfide, thus reducing the reaction resistance and improving the deposition capacity.
Bo-Quan Li, Academic Researcher, Beijing Institute of Technology
The work of the research team offers an efficient redox mediator for polysulfide reduction along with quick kinetics and confirms the application potential of sophisticated redox mediators in practical high-energy-density lithium–sulfur batteries.
Going forward, the next step will be to develop highly advanced redox mediators for full-range regulation of the cathode sulfur redox kinetics. With the help of such advanced mediators, the lithium metal anode would be safeguarded, either via the electrolyte design or by initiating lithium hosts.
The ultimate goal is to realize high-energy-density and long-cycling lithium–sulfur batteries with low costs and high safety. The potential applications of such lithium–sulfur batteries might be aerial vehicles and spacecrafts.
Bo-Quan Li, Academic Researcher, Beijing Institute of Technology
The study authors included in the research team are Yan-Qi Peng, Meng Zhao, Zi-Xian Chen, Qian Cheng, Yiran Liu, Bo-Quan Li, and Jia-Qi Huang from Beijing Institute of Technology; and Xi-Yao Li and Yun-Wei Song, from Tsinghua University.
The study has been financially supported by the National Key Research and Development Program, Beijing Natural Science Foundation, Natural Scientific Foundation of China, Scientific and Technological Key Project of Shanxi Province, and Beijing Institute of Technology Research Fund Program for Young Scholars.
Journal Reference:
Peng, Y.-Q., et al. (2022) Boosting sulfur redox kinetics by a pentacenetetrone redox mediator for high-energy-density lithium-sulfur batteries. Nano Research. doi.org/10.1007/s12274-022-4584-z.