Jeol's JMS-S3000 SpiralTOF™-plus Ultra-High Mass Resolution MALDI-TOFMS (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometer) System uses innovative SpiralTOF ion optics. The JMS-S3000 has been further developed into SpiralTOF™-plus 3.0, which now features a significantly expanded dynamic range.
This system sets a new standard in MALDI-TOFMS performance, offering cutting-edge analytical solutions for a diverse range of research fields, including functional synthetic polymers, materials science, and biomolecules.
Revolutionary, Ultra-High Performance TOF Ion Optics

Image Credit: JEOL USA, Inc.
Setting the New Standard in MALDI-TOFMS Performance
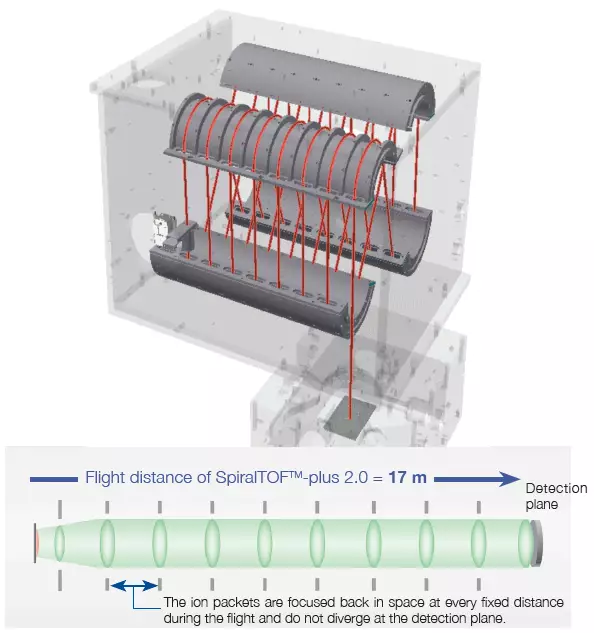
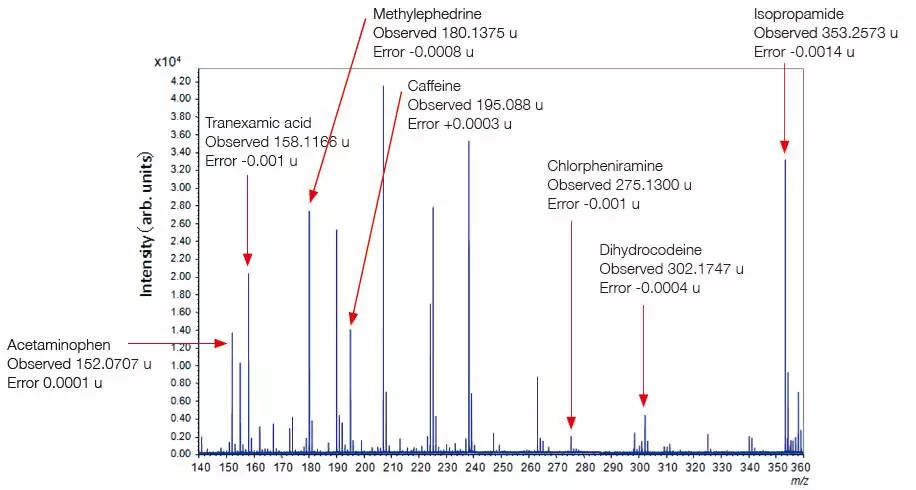
Image Credit: JEOL USA, Inc.
The mass resolving power and mass accuracy of a time-of-flight mass spectrometer can be improved by increasing flight distance, while preventing a collection of ions with the same m/z (an ion packet) from diverging in space. JEOL has created a unique SpiralTOF ion optics technology, using the "Perfect focusing" and "Multi-turn" principles to improve both values.
During flight, ion packets are focused back into space at predetermined intervals (each with a figure-eight trajectory). Despite this long distance, ion packets do not diverge at the detection plane, and a high mass resolving power, mass accuracy, and ion transmission can be obtained.
Key Features
- Monoisotopic precursor selection
- True high-energy (20 keV) CID
- Free of artifacts from post-source decay (PSD)
- SpiralTOF, TOF/TOF, and Linear TOF analyzers
- Ultra-high resolving power of >75,000 over a broad mass range
- Sub-ppm mass accuracy using an internal standard
Examples
Reduced Topographic Effect of Matrix Crystal
Differences between the matrix crystal and sample topographies can change the ions' flight start point. This results in slightly different flight times between ions that hit matrix crystals and ions that hit sample crystals.
In a traditional ion optical system, this time difference reduces mass resolving power and mass accuracy gained through external mass calibration. However, when calibrated externally, the JMS-S3000's longer flight distance minimizes this effect, resulting in highly reproducible mass resolving power and good mass accuracy.
High mass-resolution and mass accuracy can be maintained during image analysis of a biological specimen in which a large number of mass spectra are obtained over a vast region, and the specimen surface is likely to be uneven.
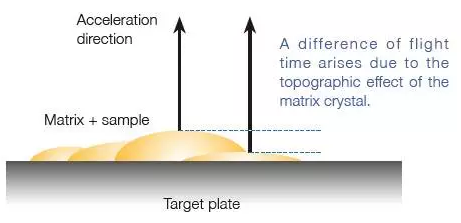
Image Credit: JEOL USA, Inc.
Achieving a Wide Dynamic Range
Implementing the SpiralTOF™-plus 2.0 improved the detection technology and resulted in a wider dynamic range in the JMS-S3000 device. This allows for peaks with ion intensity differences of up to four orders of magnitude to be simultaneously detected. In addition to traditional bulk sample measurements, the JMS-S3000's mass spectrometry imaging measurements make it easier to analyze trace components in samples.
The measurement example below shows a 1,000:1 mixture of polyethylene oxide and polypropylene oxide. Polymer analysis of samples combined with Kendrick Mass Defect (KMD) analysis allows trace components, usually difficult to identify, to be examined in sample spectra.
The Mass Spectrum of a Mixture of Polyethylene Oxide and Polypropylene Oxide in the Ratio of 1,000:1
The SpiralTOF™-plus 2.0 can analyze trace components over a wide dynamic range and calculate their molecular weight distribution.
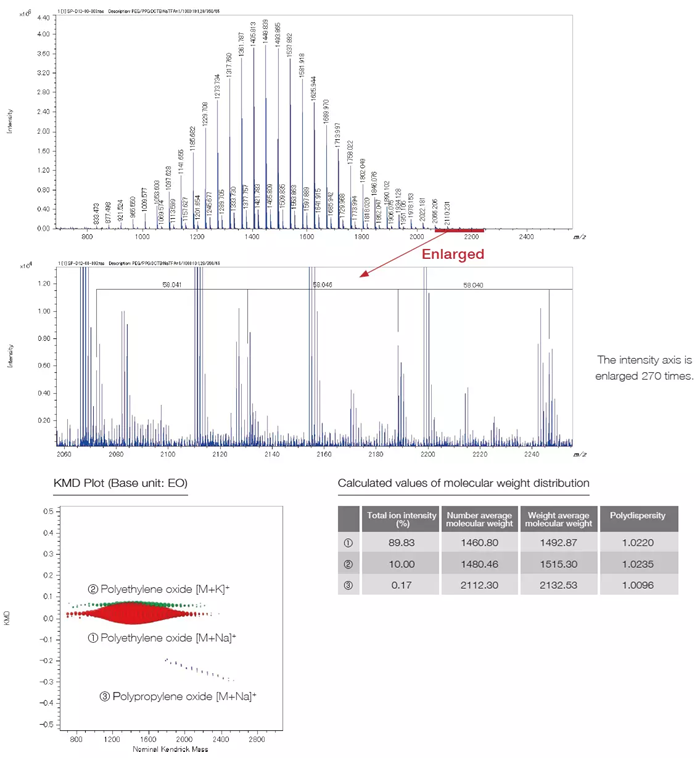
Image Credit: JEOL USA, Inc.
Features and Usages of TOF-TOF Option and Linear TOF Option
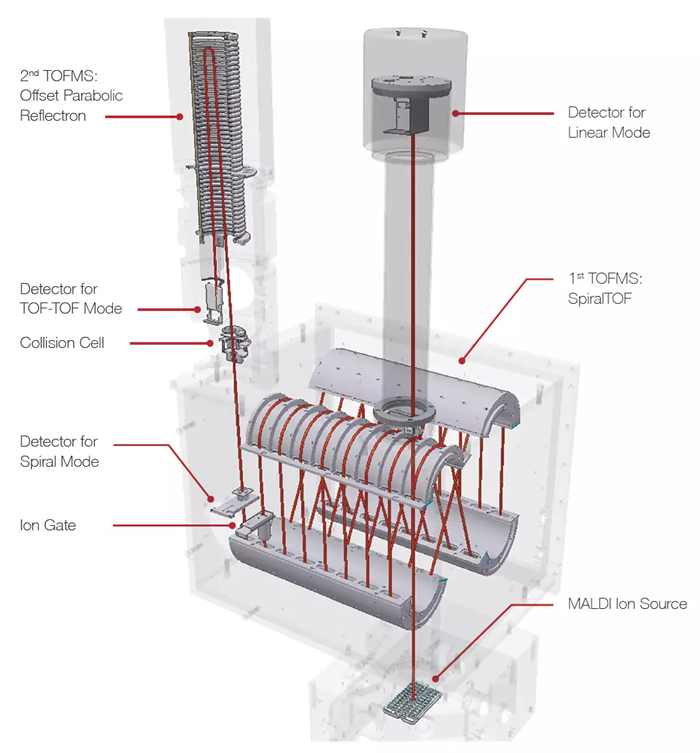
Image Credit: JEOL USA, Inc.
Features
- Excellent precursor ion selectivity can be achieved using SpiralTOF ion optics, and the monoisotopic peak of precursor ions can be accurately identified and selected.
- High-energy collision-induced dissociation (HE-CID) allows product mass spectra containing detailed structural information to be collected.
- JEOL's O-set parabolic reflectron captures all product ion information from m/z five to the precursor ion, which means the structural information obtained is reliable.
Usage
- When conducting organic compound structural analysis, composition calculations can be made more accurate by using precise mass in Spiral mode, which identifies the adduct ion in addition to the structural information received from HE-CID.
- HE-CID can differentiate structural isomers such as leucine and isoleucine when examining peptide amino acid sequences. The presence, or lack thereof, of immonium ions can confirm the presence or absence of amino acids in a peptide.
- The structural characterization of alkyl chains is essential in studying additives, surfactants, and lipids. HE-CID can be used to determine alkyl chain length and double bond positions
- When investigating polymer structure, the mass of the end groups and the ion type (adduct ion) can be confirmed using the product ion mass spectrum. Structural elucidation is further improved using the spiral mode in conjunction with the result of estimating the elemental composition.
Mass Spectra of Poly(styrene) 40K, 100K, and 200K
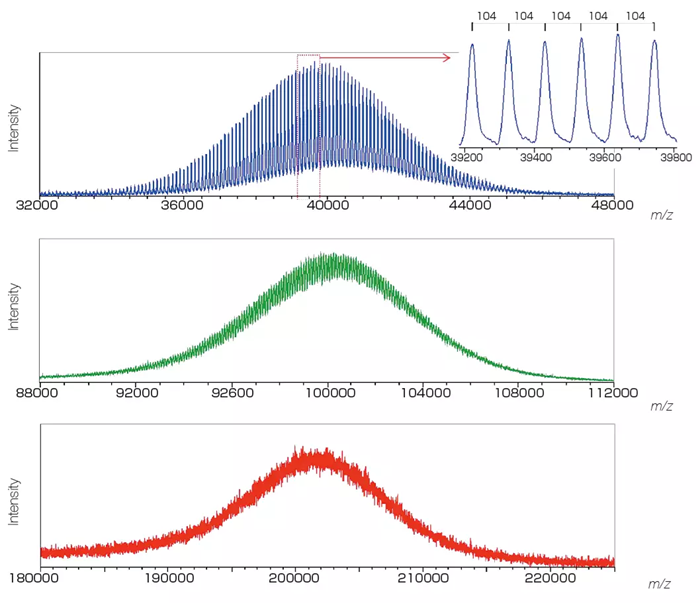
Image Credit: JEOL USA, Inc.
msRepeatFinder: The Definitive Polymer Analysis Software (Option)
SpiralTOF™-plus 2.0 is Ideal for Polymer Analysis
Industrial materials composed of polymers with distinct end groups, or copolymers, are often made up of a wide range of compounds. All components must be detected to understand their composition fully, which requires ultra-high mass resolution over an extensive mass range. It is also important to detect the baseline and trace components in samples, as different polymers and trace additives can be combined for increased polymer function.
SpiralTOF™-plus 2.0 addresses the criteria for large dynamic range and ultra-high mass resolution throughout a wide mass range. Eliminating post-source decay (PSD) generated ions, a key aspect of SpiralTOF ion optics, also helps improve mass spectrum clarity.
JEOL's MSRepeat Finder Polymer Analysis Software offers an efficient and distinctive polymer analysis solution.
Evolving Mass Imaging Analysis
MALDI MS imaging was first designed to target high molecular weight molecules like proteins and peptides. However, researchers are using it for a wider range of samples, such as to detect smaller molecules such as lipids, drugs, and pharmaceutical metabolites. Conventional MALDI-reflectron TOFMS has trouble distinguishing these small molecule signals from matrix signals, and developments are needed if these smaller mass ions are to be identifiable in spectra.
Due to its high sensitivity, signals from undesired molecules on the specimen surface frequently interfere with signals from target analytes in MALDI MS imaging. Higher selectivity is required via improved mass-resolving capabilities to obtain accurate target analyte spatial distributions.
Maintaining good mass resolution and mass accuracy over time is also required, especially when measuring a broad and uneven sample surface. SpiralTOF™-plus 2.0 is the only MALDI-TOFMS that meets imaging requirements with ultra-high mass resolution and extended flight distance, minimizing mass resolution loss due to sample surface non-uniformity. It also enables high-speed mass imaging analysis.
Mass Spectrometry Imaging Analysis of Lipids in Mouse Brain Tissue Section
Averaged Mass Spectrum of a Mouse Brain Tissue Section
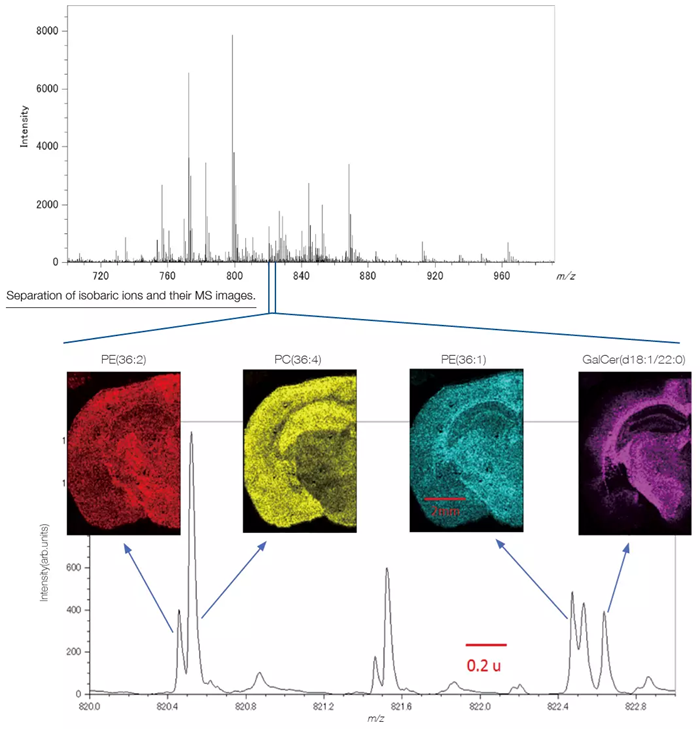
PE: Phosphatidyl ethanolamine, PC: Phosphatidyl Choline, GalCer: Galactosylceramide.The data were acquired in a joint research project with the Mass Spectrometry Group, Project Research Center for Fundamental Sciences, Graduate School of Science, Osaka University. The tissue section specimen was provided by Awazu laboratory, Division of Sustainable Energy and Environmental Engineering, Graduate School of Engineering, Osaka University. Image Credit: JEOL USA, Inc.
Mass Spectrometry Imaging of Polymers
Mass spectrometry imaging can be used for polymers. Two antioxidants, Irgafos 168 (BASF) and Irganox 1010 (BASF), were added to polymethylmethacrylate (PMMA) to create two spots. Mass spectrometry imaging was used to visualize the degradation of the UV irradiation, which was solely applied to the right spot. Polymers can show the quantitative change in both polymers and additives. Additionally, variations in the average molecular weight and polydispersity can be recorded.
MS Imaging of PMMA, Irgafos 168, and Irganox 1010:
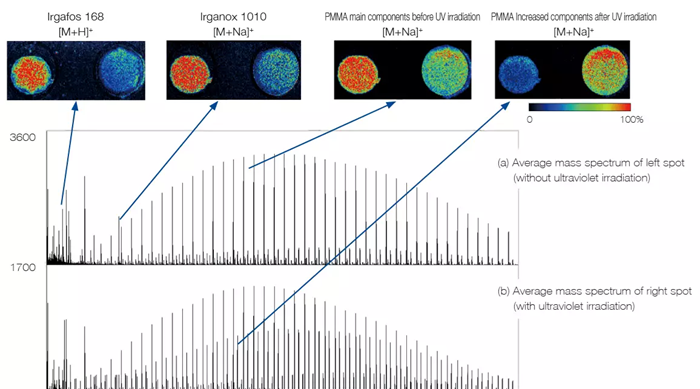
Image Credit: JEOL USA, Inc.
Analysis of Proteins
Protein identification is made possible by peptide mass fingerprinting, and in addition to the intact protein, the products of its enzymatic breakdown can also be examined for a more thorough study.
Mass Spectrum of the Tryptic Digest of Bovine Serum Albumin (BSA) and the Results of the Peptide Mass Fingerprinting:
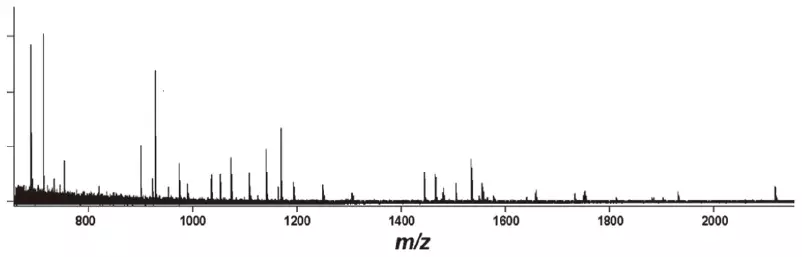
Mass spectrum of BSA tryptic digest standard (equivalent to 500 amol). Image Credit: JEOL USA, Inc.
Source: JEOL USA, Inc.
Amount
(fmol) |
Number of peptides
matched/searched |
Sequence
coverage (%) |
MASCOT
score |
| 50 |
52 / 81 |
75 |
570 |
| 10 |
41 / 79 |
64 |
390 |
| 5 |
36 / 77 |
54 |
351 |
| 1 |
28 / 57 |
43 |
255 |
| 0.5 |
31 / 52 |
46 |
306 |
| 0.1 |
12 / 34 |
18 |
92 |
* 3D-structure of RCSB PDB (www.rcsb.org) ID 1IGY (Harris, L.J., et al. (1998) J.Mol.Biol. 275: 861-872) created with Protein Workshop (Moreland, et al. (2005) BMC Bioinformatics 6:21).
Analysis of Small Molecules
Traditionally, MALDI-TOFMS techniques have been unsuitable for investigating small molecules due to matrix-derived peaks and constant chemical noise obstructing the signal from analyte molecules. However, this issue has been resolved by using SpiralTof ion optics technology.
Analysis of a Common Cold Medicine

Image Credit: JEOL USA, Inc.